Section 5.1.2: Is light a wave or a particle?
Waves and particles
One way to quantify the colours in the spectral fingerprint of each element is to use the wave description of light. While it is fairly easy to visualise water waves, or even sound waves, it is not immediately apparent what is ‘waving’ in the case of a light wave.It is in fact electric and magnetic disturbances or fields, but these are notoriously difficult to visualize.
The important point is that the distance between one crest of a wave and the next is known as the wavelength of the wave, measured in units of metres or suitable (sub-)multiples of that unit. The frequency of a wave is the number of cycles of a wave that pass a fixed point per second, measured in units of hertz (represented by the symbol Hz or s-1). Electromagnetic radiation travels at the speed of light (about three hundred thousand kilometres per second), and the speed of propagation is simply equal to the frequency of the wave multiplied by its wavelength.
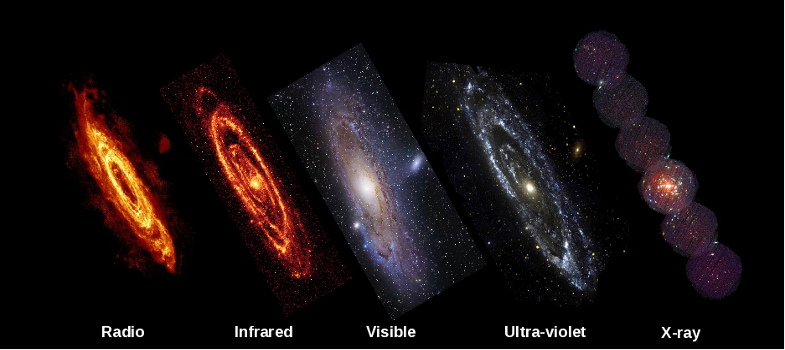
The key thing to remember is that each colour of light corresponds to electromagnetic waves of a different wavelength. Visible light spans the range of wavelengths from about 400 billionths of a metre (400 nanometres) to 700 billionths of a metre (700 nanometres). Violet light has the shortest wavelength and red light the longest wavelength. However, visible light is just one small portion of the entire electromagnetic spectrum.
ACTIVITY: Watch the video below to learn more about the electromagnetic spectrum.
(credit: National Science Foundation)
Wavelengths longer than visible light are infrared radiation, then microwaves and then radio waves, each of which spans a characteristic range of wavelengths. Infrared radiation stretches from wavelengths of less than a micrometre to about a millimetre; microwaves span the wavelength range from about a millimetre to about a metre; and radio waves may have wavelengths from less than a metre to many thousands of metres.
Wavelengths shorter than the visible are ultraviolet radiation, then X-rays and then gamma rays. The wavelengths of these types of radiation are so small they are difficult to comprehend. For example, the middle of the X-Ray range is about one nanometer (one billionth of a metre).
Although electromagnetic radiation travels from place to place like a wave, it is emitted or absorbed by matter as if it is composed of a stream of particles. These light particles are called photons. Light of a single colour consists of identical photons, each of which has exactly the same energy.
The unit of energy used to quantify a photon’s energy is the electronvolt. Photons of red light each have an energy of just less than 2 eV, while photons of violet light each have an energy of just over 3 eV. Infrared, microwave and radio photons have less energy than red light photons, where photon energies are tiny fractions of an electronvolt. Photons of higher energies include the ultraviolet, X-ray and gamma-ray regions. X-ray or gamma-ray photons have energies of many kilo electronvolts (keV, i.e. thousands of eV) or many mega electronvolts (MeV, i.e. millions of eV).
There is no conflict between the two approaches to describing light; it is just that one picture (waves) is useful for describing electromagnetic radiation propagation and another picture (particles) is useful for describing the way light interacts with matter. This double description is referred to as wave–particle duality.
ACTIVITY: Click here to learn more about wave particle duality and an experiment you can do from home to prove it!