4 Inside the atom
Before going on to see how atoms can link (bond) with each other, you need to look at atoms in a little more detail. Doubtless they are not like blocks of Lego! So what are they like?
In fact, every atom has a complex internal structure. Given the extremely small size of an atom, you may find it difficult to visualise any smaller bits inside it. However, you may already be familiar with some of the effects of one of these components - electrons. It is easy to do an experiment that shows the presence of electrons and, moreover, one of their important characteristics.
Activity 2: Detecting electrons
The items you need for this small experiment are: a plastic comb (or plastic ruler or inflated balloon) and a small piece of tissue paper or newspaper.
Tear the tissue paper or newspaper into pieces about 1 cm square and leave them in a pile on a table. Rub the comb (plastic ruler or balloon) up and down several times on your clothes. (Some materials are better than others for doing this; wool and nylon are particularly good.) Now move the comb up to the paper and note what happens.
You should find that the paper is attracted to the comb. The explanation for this phenomenon is that the rubbing action transfers large numbers of the tiny electrons from the atoms of your clothes onto the plastic comb and vice versa. The plastic builds up static electricity which attracts the paper because the electrons have an electrical charge. You now need to look at the electrons in more detail.
Each electron carries a minute but standard amount of negative charge. Conventionally, chemists and physicists speak of an electron as having a charge of −1. The units do not matter in this case as the '−1' is a comparative amount such as a ratio: one electron has a charge of −1, two electrons a charge of −2 and ten electrons have a charge of −10.
Most objects - combs, people or atoms - do not usually have any net charge. They are described as electrically neutral. This is not a very hard concept to accept in the light of some intuitive ideas from mathematics or, indeed, bank balances! Something can be negative, positive or have a value of zero, which means no charge at all.
Question 11
Atoms are neutral particles: that is, they carry no net charge. If an atom can be shown to contain negative particles (that is, electrons), what else must there be in an atom?
Answer
There must be some particles carrying a positive charge to balance the negative charge of the electrons. Moreover, the total negative charge of the electrons must just be balanced by the total positive charge in these positive particles, so that the whole atom has a net charge of zero.
These positive particles are known as protons and each one carries the same amount of charge as an electron but has the opposite sign, +1.
Question 12
What is the relationship between the number of protons in an atom and the number of electrons in the same atom?
Answer
Since they have the same charge, but opposite signs, there must be the same number of protons as electrons.
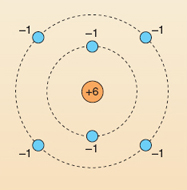
Looking at a few atoms will put this important idea into perspective. The simplest element possible is hydrogen. It has just one proton and one electron. As +1 and −1 together give a charge of 0, it follows that the atom is neutral. Next there is helium, the light but non-flammable gas that is used to fill balloons at fairgrounds and for celebrations (Figure 11).
Each helium atom contains two protons and two electrons: together, +2 and −2 equal 0. Table 3 summarises this and gives three more examples of elements that you have already met. Note, in passing, two points: first, atoms with three, four and five protons are omitted from Table 3 for simplicity: they do exist! Indeed, the 100 different elements contain atoms with, progressively, 1 up to 100 protons.
Second, most atoms also contain electrically neutral particles called neutrons. These do not greatly affect the chemistry of elements and are not discussed further in this course. They are mentioned here in case you have already heard of them and wonder why they are omitted.
| Element | No. of electrons | No. of protons |
|---|---|---|
| hydrogen | 1 | 1 |
| helium | 2 | 2 |
| carbon | 6 | 6 |
| nitrogen | 7 | 7 |
| oxygen | 8 | 8 |
The number of protons determines the identity of each element. Thus, if an atom has six protons it must be an atom of the element carbon. If it has seven protons it must be nitrogen, and so on. As the number of protons increases so the mass of the atom increases. The number of protons in an atom also determines the number of electrons in that atom and it is the protons and electrons that give the atom its unique characteristics. Note that electrons have very little mass compared with protons.
Each element has a characteristic number of protons, e.g. hydrogen has one. In a neutral atom, the number of protons equals the number of electrons.
Chemists picture an atom as comprising a central atomic nucleus, which contains the protons, with electrons moving around it. In this picture (or model) the electrons are arranged in layers, like the layers in an onion. Figure 12 shows a simple representation of this for the element carbon.
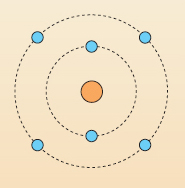
Question 13
Using Table 3 and the information in the last few paragraphs, label Figure 12 by making a note of the appropriate charge for each dot.
The nature of atoms is described above. However, in Nature very few atoms ever exist entirely on their own: most atoms are joined to other atoms by some kind of bonding. In this course we consider two ways in which atoms can bond together. Both ways depend on 'interactions' between the outermost layer of electrons of each of the atoms that are bonding. One way is called covalent bonding (pronounced 'co-vay-lent') and the other is called ionic bonding (pronounced 'eye-on-ic'). They are discussed in Section 5 and Section 7 respectively.
Question 12
The nucleus of each atom of the element gold contains 79 protons. How many electrons are there moving around each atomic nucleus in this element?
Answer
A gold atom has 79 electrons. Atoms of gold, like atoms of all other elements, are electrically neutral. The charge carried by a proton is +1 so the charge on the nucleus of a gold atom is +79. To balance this, there must be 79 electrons each with a charge of −1.
Question 13
Totally dry air, from which all carbon dioxide has been removed, contains the following gases - in decreasing order of concentration:
Nitrogen (7), oxygen (8), argon (18), neon (10), helium (2), krypton (36) and xenon (54). (Note: krypton is pronounced 'krip-ton', and xenon is pronounced 'zen-on'.)
All these gases are elements. The figures in brackets are the number of electrons in each kind of atom. How many protons are there in the nucleus of each kind of atom? How did you deduce these values?
Answer
There are seven protons in the nucleus of a nitrogen atom. This can be deduced as follows: as there are seven electrons surrounding the nucleus in a nitrogen atom, the total negative charge is −7. Since the atom is electrically neutral, and the charge on a proton is +1, there must be seven protons altogether.
Similarly, the number of protons per atomic nucleus in each of the other elements is: oxygen, 8; argon, 18; neon, 10; helium, 2; krypton, 36; and xenon, 54.
Note, for interest, there is nearly 1% of argon in the air you breathe.