5 Molecules and covalent bonding
Covalent bonding is one kind of linking that joins atoms together. The group of atoms held together by covalent bonds is a molecule. The example you are most familiar with is the compound water: water consists of covalent molecules, i.e. it is a covalent compound. Recall what is in molecules of water from Section 2.5.
Question 14
Which atoms are in a water molecule? How are they bonded together?
Answer
A water molecule comprises two hydrogen atoms and one oxygen atom, and they are bonded covalently. (Look back at Figure 8.)
You have met three other compounds in the text so far: protein (Figure 10), methane (Question 10) and carbon dioxide (Question 11). These all involve covalent bonding and all exist as molecules. You have also met some gaseous elements that exist as covalent molecules. The oxygen gas in the air does not exist in the form of free individual oxygen atoms, but as pairs of oxygen atoms joined together by covalent bonds to give oxygen molecules. The same applies to the nitrogen in the air: here two nitrogen atoms join together to form a covalent molecule. (As noted earlier, very few elements exist as free, solitary atoms.)
Before going on, make sure that you are clear about three crucial points concerning molecules.
Key points about molecules
It is possible to have molecules that are elements (e.g. a molecule of oxygen) and molecules that are compounds (e.g. a molecule of water).
Molecules always consist of two or more atoms bonded together (e.g. two oxygen atoms bond together to make an oxygen molecule; two hydrogen atoms and one oxygen atom bond together to make a water molecule).
The kind of bonding in molecules is always covalent. If a compound or an element exists as molecules, the bonding has to be covalent.
Now consider the number of covalent bonds that different atoms like to form.
Question 15
The element nitrogen exists as a covalent molecule. From what you have read above, what is in a molecule of nitrogen?
Answer
Two nitrogen atoms joined together covalently.
Question 16
Nitrogen molecules and methane molecules are both covalent but one is an element and the other is a compound. Why is one described as an element and one as a compound?
Answer
Only one kind of atom is involved in the nitrogen molecule: nitrogen atoms. Two kinds of atom are involved in a methane molecule: carbon and hydrogen.
Look back at Figure 10. This is just part of a molecule of a protein. Recall that there are only four kinds of atom involved in a molecule of a protein: carbon, nitrogen, oxygen and hydrogen. The black lines in Figure 10 represent the covalent bonds. A protein molecule is always very large, and the dotted lines represent covalent bonds going to parts of the molecule not shown in the diagram. This may look very complex, but atoms obey fairly strict rules as to how they interconnect with other atoms. In particular, there is almost always a set number of covalent bonds that a given atom can form.
In the rest of this section, models of some of the more common atoms are used to show how more complex molecules, such as the protein in Figure 10, can be built up from simple atoms. This will show you how many different kinds of molecule can be built up from the same set of atoms; in short, from the chemists' Lego set!
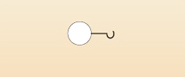
Start by looking at the simplest atom. You have already seen that this is hydrogen and that it has one proton and one electron. Hydrogen likes to form just one bond with another atom. Visualising the bonding between atoms can be very difficult - unless, once again, a model is used. This time sketches of the different atoms somewhat similar to those used in the protein molecule in Figure 10 will be used, except that instead of straight lines hooks will be used. Thus, you might represent hydrogen as a sphere with one hook since it has one bond, as shown in Figure 14.
When linking atoms together to make molecules, the 'golden rule' is that no atom must ever have any spare hooks. A hydrogen atom all by itself has got a spare hook and that is not allowed.
Question 17
What is the simplest molecule that hydrogen atoms alone can form? Use representations of the hydrogen atom, such as that in Figure 14, to sketch the molecule.
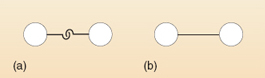
Chemists usually draw the links between the different atoms that form molecules in the form of straight lines. This is shown for hydrogen in Figure 15(b). By comparing (a) and (b), you can see that 'two linked hooks' equals 'one covalent bond'.
Now apply the model-building idea to a molecule of water. Oxygen has two hooks, as shown in Figure 16.
Question 18
Sketch a representation of the water molecule, but this time leave out the 'joined hooks' stage and write down the straight lines of the covalent bonds.
Answer
Your answer should look similar to that shown in Figure 8(a).
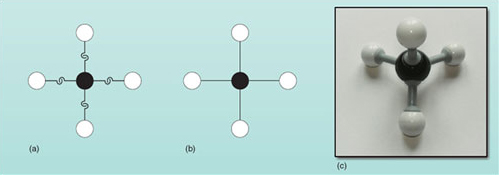
Now consider something slightly more complicated than a hydrogen molecule or water molecule. Methane, which is used in domestic heating and cooking, is a covalent compound that has been mentioned several times before. A molecule of methane contains only carbon and hydrogen. In fact, the molecule contains just one atom of carbon. Carbon atoms have four hooks as shown in Figure 17.
Question 19
Use the model atoms of hydrogen (Figure 14) and carbon (Figure 17) to obtain a representation of the methane molecule. How many hydrogen atoms can be attached to the one carbon atom?
Answer
Your model should look similar to Figure 18(a). Each of the four carbon hooks attaches to a hydrogen hook to produce a methane molecule in which four hydrogen atoms form bonds to one carbon atom. Figure 18(b) shows the same molecule using bonds; as before, two joined hooks equals one covalent bond. Figure 18(c) illustrates a ball-and-stick model of methane.
Note that methane isn't a flat molecule - it is described as tetrahedral in shape, as shown in the ball-and-stick model (Figure 18(c)). (The four hydrogen atoms form the four corners of a tetrahedron with the carbon atom at the centre.) However, this is difficult to draw, hence chemists often don't accurately depict the three-dimensional shapes of molecules in written representations.
Try another example: carbon dioxide. This is the molecule produced when carbon (in coal, wood or oil) is burned and when humans or animals breathe out. The name of a compound sometimes gives useful information. In this instance, the 'di' in front of the oxide of dioxide tells you that the molecule has two oxygen atoms. The carbon dioxide molecule demonstrates another feature of bonding between atoms.
Question 20
How many bonds does carbon form? Look back at Figure 17 if necessary.
Answer
Carbon forms four bonds.
Question 21
How many bonds can oxygen form? Look back at Figure 16 if necessary.
Answer
Oxygen forms two bonds.
So, how does one carbon atom bond to two oxygen atoms in this instance? Imagine that all the hooks sticking out of the spheres of the atoms of carbon and oxygen are flexible. Try to fix them together so there are no unsatisfied hooks.
The only way for all the carbon hooks to be used is (i) for the two hooks of one oxygen atom to link to two of the four hooks of the carbon atom and (ii) the second oxygen atom to link in the same way to the remaining two hooks of the carbon atom. The molecule is represented in Figure 19(a) .
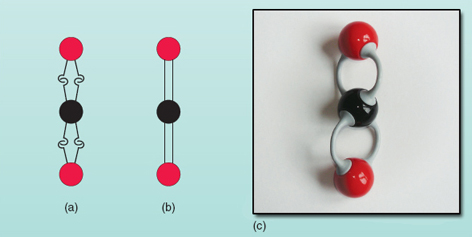
This type of sketch is quite clumsy and chemists prefer to represent the bonds as shown in Figure 19(b). When atoms bond in the way shown in this figure, the bonds formed are referred to as double bonds, as opposed to single bonds such as those formed in the methane and water molecules. Look back at Figure 10 - part of a protein molecule. The vertical 'double lines' between the carbon atoms and the oxygen atoms are carbon-to-oxygen double bonds.
It is entirely possible to have other molecules where carbon atoms are joined to carbon atoms by double bonds - as shown in the example in Figure 20. (Note: Chemists have a special name for compounds that contain carbon-to-carbon double bonds. They are described as unsaturated compounds or sometimes as unsaturates. If there are several such double bonds in a molecule, they are often called polyunsaturates, where 'poly' simply means 'many'. You may have heard the term in expressions such as 'high in polyunsaturates' used to describe certain margarines and spreads.)

The number of covalent bonds that are normally formed by hydrogen, carbon, nitrogen and oxygen (the four atoms found in molecules of protein) are summarised in Table 4. To extend your 'chemistry Lego set' a little further, two other elements have been added to the table, namely sulfur and chlorine.
| Element | Usual number of bonds |
|---|---|
| hydrogen | 1 |
| carbon | 4 |
| nitrogen | 3 |
| oxygen | 2 |
| sulfur | 2 |
| chlorine | 1 |
Footnotes
You may find it helpful to memorise this table.In concluding this section on covalent bonds, it is important to remember that it is not really hooks that hold atoms together! You learned from Figure 12 that the nucleus of every atom is surrounded by electrons. When two atoms link covalently some of these electrons are shared between them. This idea of 'electron sharing' in covalent bonds is an important one in chemistry.
Question 14
Using the information in Table 4, which of the following are likely to exist as covalent molecules?
(a) One sulfur atom and two hydrogen atoms;
(b) One nitrogen atom and three hydrogen atoms;
(c) One carbon atom and five chlorine atoms.
Answer
(a) and (b) are likely to exist and in fact do exist as covalent compounds; (c) is most unlikely as carbon forms four and not five covalent bonds.