3 What are compounds?
Activity 1: Elements and compounds
Click on the video clip to watch Elements and Compounds, which focuses on water and its constituent elements.
Click below to view video clip. (6 minutes)
Transcript: Video 1
Although there are about only 100 elements, there are many, many more than 100 substances in the world - not just thousands but millions of different substances. You could begin a list starting in your kitchen: water, salt, sugar, vinegar, bicarbonate of soda. None of the substances in this particular list are elements, so what are they? They are substances in which atoms of different elements are joined together. The proper chemical term for any such substance is chemical compound or just compound.
Question 8
Is water a compound or an element?
Answer
Water is a compound. It contains more than one element: hydrogen and oxygen atoms are joined together; as illustrated in the video clip Elements and Compounds, above.
An important feature of compounds is that they are very different from the elements from which they are made. For example, water is made from hydrogen and oxygen, which are both colourless gases, whereas water is the wet liquid you drink that makes up 65% of your body. So, it is important to realise that a water molecule is quite different from the two types of atom from which it is formed. Water is not simply a mixture of hydrogen and oxygen; it contains hydrogen and oxygen atoms linked together in an ordered way. (You can make a house from Lego but you would not look at a pile of the separate blocks and say that is a house! In scientific terminology, the house is the molecule and the blocks from which it is built are the atoms.)
Question 9
Look back at Table 2. From what you know about the composition of living organisms, why do you think the percentages of hydrogen and of oxygen atoms are so great?
Answer
If 65% of human bodies is water, you would expect to have a high percentage of the elements that make up water (hydrogen and oxygen) in your body.
The next most common element in human bodies, after hydrogen and oxygen, is carbon. This, when linked to other atoms, forms most of the compounds of which plants and animals are made (apart, that is, from water). One very important category of compounds found in plants and animals is the proteins. Part of a protein molecule is shown in Figure 10. This very large molecule (it contains thousands of atoms!) is made up of only four different types of atom; note the complex way in which the atoms are put together.
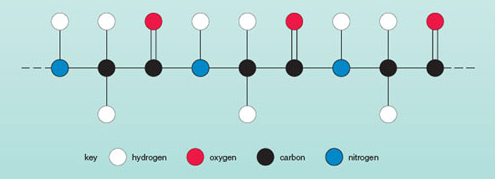
Question 10
Using the key in Figure 10, name the different kinds of atom (hence different kinds of element) found in a protein molecule.
Answer
Carbon, nitrogen, oxygen and hydrogen atoms are found in protein molecules.
Therefore, it is possible to have simple molecules such as water where only three atoms are bonded together to make a water molecule, and very complex molecules such as proteins where very large numbers of atoms are bonded.
Practise your understanding of elements and compounds by trying the following questions.
Question 9
Using the information about the types of atoms in water and protein (Figures 8 and 10), which of the following are elements and which are compounds?
hydrogen; water; nitrogen; carbon; protein
Answer
Water and protein are compounds because they consist of different types of atom bonded together. Hydrogen, nitrogen and carbon are elements because they each consist of only one type of atom.
Question 10
The gas methane is a major constituent of the gas used for cooking and heating. The only kinds of atom present in a molecule of methane are hydrogen and carbon. Use your understanding of the earlier parts of this course to complete the blanks in the following correct statement.
Methane contains the ……………… carbon and ……. Methane is not an element. It is a chemical ………………
Answer
Two answers are equally correct. Either fill in the blanks with elements/hydrogen/compound or with atoms/hydrogen/compound.
Question 11
The atmosphere contains several different kinds of gas: about 80% is nitrogen and about 20% is oxygen. There is a small amount of other gases, one of which is carbon dioxide. From the information given in statements (a) and (b), decide whether the gas named in each statement is an element or a compound.
(a) The bubbles of gas produced in beer and wine making are pure carbon dioxide. Analysis shows that the bubbles contain molecules in which there are two kinds of atom bonded together, namely carbon and oxygen.
(b) In nitrogen gas, nitrogen atoms are bonded together in pairs.
Answer
(a) Carbon dioxide is a compound; (b) nitrogen is an element.
