5 Crystal defects and twinning
Virtually all crystals contain minute imperfections or defects. The effect of defects on the physical and chemical properties of a crystal can be out of all proportion to their size.
Defects come in several types. Point defects may involve missing or displaced atoms in the crystal structure, giving empty sites, or vacancies. Such defects make it much easier for atoms to diffuse through the crystal structure by moving between vacant sites. This is important because the rate of diffusion of atoms through a crystal structure can determine the speed at which processes such as weathering, or other chemical reactions (e.g. during metamorphism) proceed.
Minerals in deformed rocks, such as those from mountain belts, contain large numbers of line defects caused by rows of atoms that are out of place in the crystal structure. Figure 22 shows an artificial example based on an alloy used in semiconductors, where each dark line represents a strained part of the crystal. Such defects affect the mechanical strength of a crystal, which determines the strength of rocks and how they deform under intense pressure. During deformation, progressive movement along flaws in crystals takes place in tiny steps, as bonds are broken and re-formed.

A third type of defect is called a planar defect. Crystals grow by the progressive addition of atoms onto a surface. 'Mistakes' in the stacking of new planes with respect to previously formed planes are common during crystal growth. These planar defects can have a profound effect on the way atoms are stacked, and can produce distinct regions called domains within a single crystal. One such planar defect is a boundary that separates two domains of a crystal that are mirror images (Figure 23). The result is called a twinned crystal.
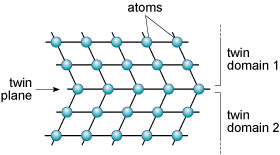
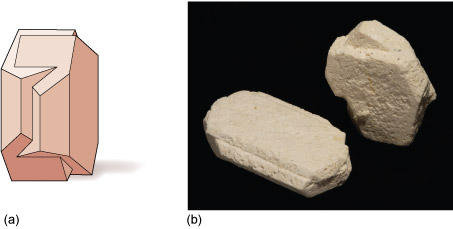
Various types of crystal twinning exist, and in each case a single crystal consists of two or more regions with different orientations. The different regions of the twinned crystal may be related in various ways, such as by rotation about a symmetry axis (Section 6.2). Twinning is especially common in feldspar. Orthoclase feldspar often displays simple twinning, in which the crystal is divided into two domains with a different structural orientation (Figure 24).
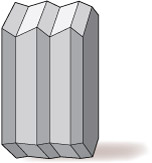
Other types of feldspar (e.g. plagioclase feldspar) may have a more complex type of twinning, called multiple twinning, in which a single crystal has many different domains (Figure 25).
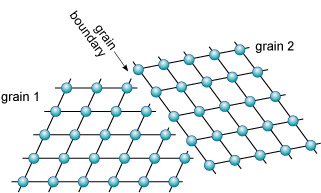
Grain boundaries are different from twin boundaries because there is no orientation relationship between crystals on either side of the grain boundary. There are two distinct grains, with the same, or a different, mineral composition (Figure 26).