3.2 Interactions of light and matter
Light, or visible radiation, interacts with parts of our eyes and is translated by our brains into the perception of colours.
Question 8
Using Figure 5, what is the approximate wavelength of the visible region of the electromagnetic spectrum and which regions lie on either side of it?
Answer
The 'visible spectrum' is radiation with a wavelength of about 500 nm and it lies between the infrared and the ultraviolet regions of the spectrum.
Wavelengths starting at about 7 x 10-7 m (700 nm) are perceived as the colour red. As the wavelength decreases to about 4 x 10-7 m (400 nm), the colour we perceive changes from red through to violet. Daylight is composed of all of the radiation in the visible region, which results in 'white' light.
When light, or any other form of electromagnetic radiation, interacts with any kind of matter, including living matter, the light can be:
- reflected
- absorbed
- transmitted (pass through unchanged)
- absorbed at one wavelength and emitted at another (longer) wavelength.
These phenomena are exploited in many ways in forensic science and analytical science generally. They are especially useful because different materials will interact highly specifically with radiation of different wavelengths.
If you are wearing a blue piece of clothing and are looking at it in daylight, the principles of the scientific use of radiation can be seen. When the daylight (visible radiation) hits your blue clothes, all (or most of) the colours except blue are absorbed (by the blue dye) and the blue light is reflected back into your eyes (Figure 6).
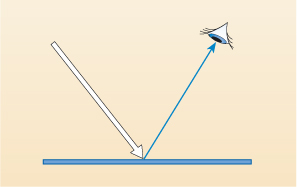
Different-coloured materials absorb and reflect different wavelengths. Consider what happens when a piece of coloured glass is held in front of your eyes.
Question 9
If that coloured glass appears to you to be blue, what do think is happening to the daylight that is falling on it?
Answer
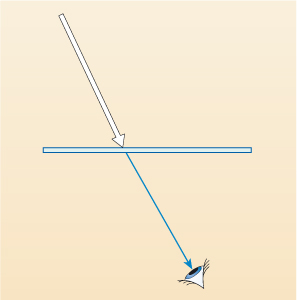
All of the different wavelengths except blue are either absorbed (or possibly reflected back) and the blue light passes through - is transmitted - and is detected by your eyes, as shown in Figure 7 below.
The process by which light is absorbed at one wavelength and emitted at another, longer wavelength is called fluorescence. This principle is used in 'optical brighteners' in washing powders. These emit light in the blue region of the visible spectrum which reduces the appearance of 'yellowing' in white material and the emitted light increases the amount of light reaching the eye, making white garments washed in them appear bright and white.
Question 10
In order that they can emit blue light, which wavelengths will the optical brighteners need to absorb?
Answer
The absorbed light must have a shorter wavelength than visible blue light, which, from Figure 5, is ultraviolet radiation. (You may already know that sunlight contains some ultraviolet (UV) radiation. It is that which causes sunburn if the skin is exposed to sunshine for too long.)
So, by a complex process, the molecules of the optical brighteners absorb radiation in or near the ultraviolet region. Some body fluids can also fluoresce, as you will see later.
These interactions of electromagnetic radiation with matter - reflection, absorption, transmission and fluorescence - are exploited in many ways in forensic science in a set of techniques collectively called spectroscopy.
In spectroscopy, matter is exposed to radiation and the pattern of absorption, transmission, reflection or fluorescence emission of this radiation by the material is measured and interpreted. The patterns obtained can reveal a great deal about the chemical and physical structures of substances.
In fingerprint analysis the observation of fluorescence can be used to visualise some fingerprints that are otherwise invisible to the eye. This technique involves shining ultraviolet radiation onto the sample. Since biological fluids, such as semen, saliva, urine and others fluoresce, and any of these fluids could find their way onto the fingertips that leave fingerprints at crime scenes, an 'invisible' or latent fingerprint may be revealed by fluorescence (Figure 8). It can then be photographed for later comparison with prints from suspects and others.

