4.3 Bicarbonate
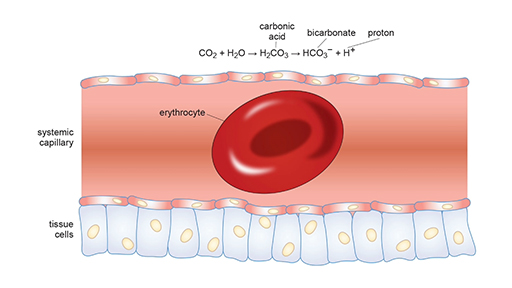
In the previous section, you saw how the affinity of Hb for O2 decreases in the presence of elevated CO2 and acidity. This is known as the Bohr effect. This is due to the chemical reaction that takes place between CO2 and water (H2O) to generate bicarbonate (HCO3−) and protons (H+). This reaction is represented by the equation:
In chemistry, the ⇌ arrow represents a reversible reaction, meaning it can go in the right or the left direction. In this case, adding more CO2 will push the reaction to the right and generate more H+ and HCO3− ions. H+ ions decrease the pH of a solution (make it more acidic) whereas HCO3− ions increase the pH and make it more basic.
The reversible nature of this reaction is critical in allowing the body to transport CO2 from the tissues and be exhaled in the lungs. This process is detailed in Video 12.
Transcript: Video 12 Bicarbonate buffering.
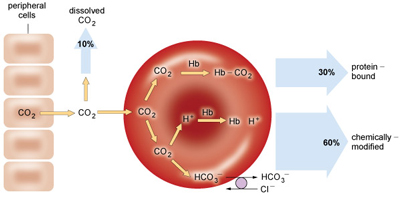
In Video 12, you saw that protons (H+) generated during bicarbonate buffering of CO2 bind to Hb in the erythrocytes to form protonated haemoglobin (HbH+). This binding decreases the affinity of Hb for O2, thereby facilitating O2 diffusion into tissues, as described by the following equation:
At the same time, CO2 that has not been converted into HCO3− (~30% of total CO2 in the blood) binds with high affinity to deoxyhaemoglobin to form carbaminohaemoglobin (HbCO2). This complex is then carried to the lungs (Figure 12).
In the alveoli, binding of O2 to HbH+ results in the release of free H+ ions.
Question 10 Higher H+
a.
left, towards increased CO2 production
b.
right, towards HCO3− production
c.
neither, the reaction will stay in equilibrium
The correct answer is a.
Answer
The answer is left. It will help drive the diffusion of CO2 out of the blood and into the alveoli to be exhaled.
In parallel, carbaminohaemoglobin loses its affinity for CO2 as it becomes reoxygenated. Collectively, these actions increase the PCO2 at the alveoli. The phenomenon by which O2 influences CO2 concentrations is known as the Haldane effect.
The capacity of the blood to carry O2 is also greatly reduced by carbon monoxide (CO), a gas emitted by car exhausts and faulty gas appliances. CO competes with O2 for binding to Hb. Because the affinity of Hb for CO is higher than its affinity for O2, CO molecules will bind preferentially and irreversibly to form carboxyhaemoglobin (HbCO), which is cherry red in colour. Inhaling CO will therefore progressively reduce the amount of Hb available to bind O2 and lead to CO poisoning. If the source of CO is not removed, death could result due to the total lack of oxygen (asphyxiation).