3 A metal’s signature
In this section you are going to investigate how metals can be identified by the colour they impart to a flame. Electrons are found in shells around the nucleus. These shells are numbered 1, 2, 3 (and so on), moving outward from the nucleus (Figure 7). The number of the shell is known as the principal quantum number, and is given by the symbol n.
An electron’s energy depends on the shell it is in; in general as n increases, the energy of the electron also increases. The energy level occupied by electrons depend on the amount of energy in the system. At high temperatures (such as under a flame), electrons in the metal atoms will absorb heat energy and be promoted to higher energy levels.
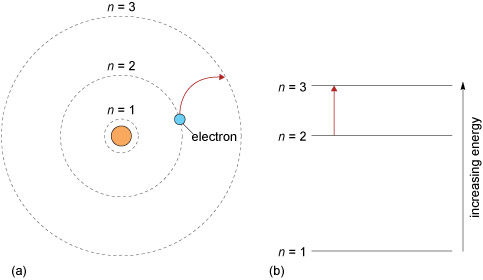
The colour of the flame arises when these excited electrons return to lower energy levels emitting energy as light of a characteristic frequency. This gives a characteristic colour to the flame when a metal is heated in it (Figure 8). If you have ever let a pan containing salt in water boil over on a gas stove you may have noticed that the flame goes yellow; this is caused by the electrons in the sodium atom.
Visible light can be split by a prism into an uninterrupted band of colours, known as a continuous spectrum. However, the spectrum produced by the excited electrons of a particular element falling to lower energy levels consists of discrete coloured lines in a dark background. In Figure 9 you can see the emission spectrum of sodium when a beam of light from a sodium lamp is dispersed by a prism; the two intense yellow lines emitted by sodium atoms, are the main reason for the flame colour shown above.
Each element produces a unique set of spectral lines, and in the next section you will use the emission spectra of elements to identify some metals.


