2 Arrangement of atoms in metals
Most metal atoms pack closely together in a similar way to fruit on packing trays (Figure 2). This arrangement is the most efficient use of space as apples are packed as closely together as they can. Note how most of the apples are in contact with six neighbouring apples.
But metals are three-dimensional. The layer of atoms represented by the apples in Figure 2 will be covered by other layers in a solid metal. For most metals, this three-dimensional structure can be seen in the way fruit is sometimes stacked on market stalls. A second layer of apples will fit neatly in the hollows created by the bottom layer. The third layer of apples will lie directly above the first layer. This pattern is known as a hexagonal closed packed (hcp) structure. Watch Video 1 which illustrates this structure using spheres to build a model.
Transcript: Video 1 Close packing in three dimensions. (2:46 min)
The ongoing arrangement of metal atoms in a three-dimensional regular ordered pattern is known in chemistry as a lattice structure.
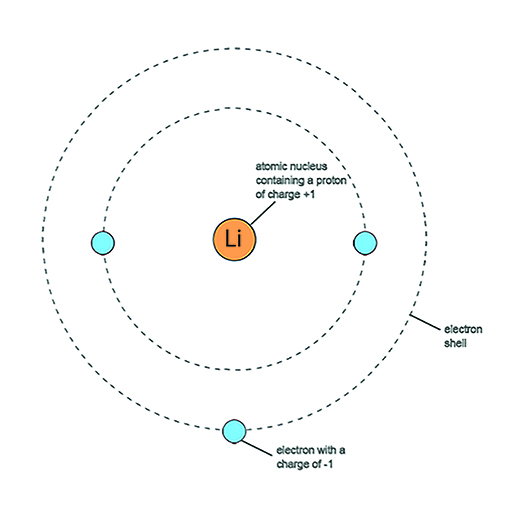
A simple model of an atom is a central atomic nucleus with electrons arranged in shells at different distances from the nucleus. Lithium has the smallest atom of all metals. Figure 3 shows a simple representation of a lithium atom. The smaller dots represent electrons moving around the central nucleus. The large dot at the centre represents the nucleus of the lithium atom containing protons and neutrons. Moving outwards from the nucleus, you can see a region of empty space before a narrow region where there is a good chance of meeting a set of two electrons. This first shell can only contain a maximum of two electrons. Then there is more empty space before another narrow region in which up to six electrons can be found.
Each electron carries a minute but standard amount of negative electric charge, or charge for short. Electric charge is the property of matter that causes electrical phenomena. Conventionally, chemists and physicists speak of an electron as having a charge of −1. The units do not matter in this case as the ‘−1’ is a comparative amount: one electron has a charge of −1, two electrons a charge of −2 and ten electrons a charge of −10.
However, atoms are neutral particles: that is, they carry no net charge. This means that the total negative charge of the electrons must be balanced by the total positive charge in these positive particles in the atom, so that the whole atom has a net charge of zero. These positive particles are known as protons and each one carries the same amount of charge as an electron but has the opposite sign, +1.
Each element has its own specific signature in terms of number of protons, neutrons and electrons. Lithium has 3 protons, 4 neutrons and three electrons.
-
What overall charge would an atom of lithium have?
-
One atom of lithium would have an overall charge of zero as the three positive protons will equal the negative charge of the three electrons.
-
If a nucleus of an atom was separated from the rings of electrons would it have a positive or a negative charge?
-
The nucleus would be positively charged because of the protons within it. The strength of the positive charge would depend upon the number of protons within the nucleus.
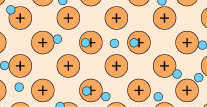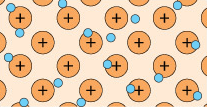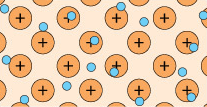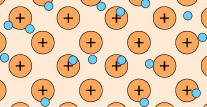
A feature of metal atoms is that the electrons in the outer shells do not remain in the proximity of a specific nucleus. In bulk metals, these electrons, rather than being associated with any particular metal atom, can be thought to be part of a shared ‘sea’ of electrons that move freely (Figure 4). These are known as delocalised electrons.