2 Environmental regulation of physiological processes
2.1 Nutrient budgeting
All plants and animals respond to environmental changes such as the light–dark cycle and temperature, but the impact of the environment on essential physiological processes such as eating, fattening and breeding is more evident and often more finely controlled in polar species than in those that are native to warmer and more equable habitats. Large effects are nearly always easier to quantify and to investigate experimentally, so arctic species offer an excellent opportunity to study the subtle but often important action of environmental changes on physiological processes.
A good place to begin such an analysis is with nutrient budgeting. Energy is expended in the search for food, and in ingesting and digesting it. If food is so scarce that searching is inefficient, or its nutrient content so low that little nourishment is obtained from it, animals may be able to save energy by suppressing appetite and fasting. In polar environments, food is widely scattered both in space and in time. Consequently, the physiological mechanisms that regulate appetite and energy storage are sophisticated and effective in arctic species. Herbivorous animals such as reindeer are directly dependent upon plant productivity and synchronize their foraging and other energetically expensive activities, such as mating and breeding, with it. Daylength (photoperiod) is a more reliable indicator of season than temperature (see Figure 1) and is often an important regulator of physiological mechanisms.
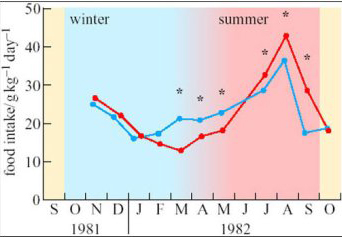
To investigate seasonal changes in the behaviour and metabolism of species native to the high Arctic, a few adults of the subspecies of reindeer that is endemic to Svalbard (Rangifer tarandus platyrhynchus, see Figure 2b) were transported to northern Norway and kept in small outdoor pens there, alongside similar individuals of the native subspecies, Rangifer tarandus tarandus (Larsen et al., 1985). All the animals had continuous, unrestricted access to forage but, as shown on Figure 3, the Svalbard reindeer ate three times as much food in August as in March.
SAQ 1
Are these seasonal changes in the appetite of Svalbard reindeer simply a direct response to the environment?
Answer
No. There were seasonal changes in the food eaten by local Norwegian reindeer as well, but they were less pronounced than those of the animals native to high latitudes. In addition, the largest differences between the two subspecies were observed in mid-March and mid-September, around the equinoxes when day and night are equal in length over the whole globe.
SAQ 2
Do seasonal differences in energy expenditure explain these data?
Answer
No. Being confined in small pens, the reindeer took little exercise all the time. Energy expended on thermoregulation should be greater in cold weather, so if thermogenesis was important, one would expect them to eat more, not less, in the winter.
Reindeer (Figure 2b) grow thick coats of long, hollow hair that insulates the warm skin so effectively that snow accumulates on their backs without melting. Energy expenditure on shivering or other forms of thermogenesis seems to be minimal even in the coldest weather. Foraging is slower and less efficient in winter, and the lower total daily intake is supplemented by utilization of the fat reserves built up during the brief summer, when they eat almost continuously. However, as these experiments show, the seasonal changes in food intake arise primarily from the endogenous control of appetite, and are not imposed upon the animals by food availability. The fine control of appetite is slightly different in subspecies adapted to different climates. The investigators also found small but significant differences at certain times of year between Norwegian and Svalbard reindeer in the rates of lipogenesis measured in adipocytes in vitro, and in the responses of adipose tissue to hormones such as adrenalin.
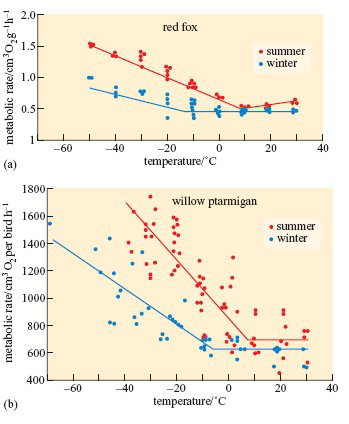
Metabolic rate, food intake and other aspects of energy balance also change seasonally in birds and mammals that are native to high latitudes. The red, or common, fox (Vulpes vulpes) occurs throughout Europe and northern Asia except in high mountains and arctic regions, where it is replaced by the smaller arctic fox, shown in Figure 2a. As shown on Figure 4a, at above 10° C, the fox's basal metabolic rate (BMR) is about the same in summer and winter, but as the temperature falls, the rise in BMR is delayed and is slower in winter-adapted animals than in those caught in summer.
Such phenomena have been intensively investigated in ptarmigan (Figure 5) which are non-migratory, mainly ground-dwelling grouse-like birds that eat twigs, shoots and other plant material. There are two species in Scandinavia and Russia: the willow ptarmigan (Lagopus lagopus lagopus;Figure 5) and the rock ptarmigan (L. mutus mutus). (‘Lagopus’ means ‘foot of a hare’ and refers to the feather-covered or fur-covered feet of the ptarmigan and arctic fox, see Figures 2a and 17.)

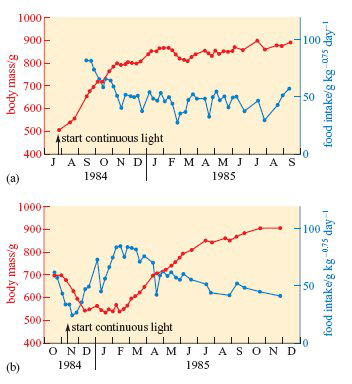
A subspecies of rock ptarmigan occurs only on Svalbard; it is larger than the mainland forms, and has almost pure white plumage during the 8 months of winter. As shown on Figure 4b, the metabolic rate of willow ptarmigan measured at a wide range of temperatures is lower in winter than in summer. The seasonal differences are even greater in Svalbard ptarmigan (L. mutus hyperboreus). Svalbard ptarmigan also eat much more in the late summer than in winter and accumulate fat in the autumn. The experiments summarized in Figure 6 reveal some of the physiological mechanisms that control these changes in appetite and energy storage (Lindgård and Stokkan, 1989).
When exposure to continuous light was started in July (Figure 6a), the birds’ usual autumnal fattening proceeded as normal, but their body mass remained high and food intake fairly low, right through to the following September.
Throughout this period, their plumage remained white and they failed to breed. It was as though the continuous light held them indefinitely in their autumnal condition. However, when exposure to continuous light was started in November (Figure 6b), the birds underwent a complete cycle of changes in body mass and food intake (and began to develop speckled summer plumage) before settling into continuous high body mass and low appetite.
SAQ 3
What do these experiments show about how seasonal changes in appetite and body mass are controlled?
Answer
They are not simply a response to environmental conditions but are at least partly controlled endogenously.
Exactly how such control mechanisms evolved and what happens when animals (or people) are abruptly transported into environments in which their endogenous controls of appetite and energy expenditure are inappropriate are not known.
Although several ruminant mammal species live in mountains and arctic regions (e.g. mountain goats and reindeer, respectively), none is known to hibernate in the strict sense of the term. There are no living species of the family Bovidae smaller than sheep or goats, but some deer (family Cervidae) are less than a tenth of that size as adults. Some tropical deer, notably species of mouse deer Tragulus, weigh only 1–2 kg, well within the range of size of mammals that can become torpid, but none is known to do so.
One reason might be that substantial changes in body temperature would kill the microbes in the rumen that are essential to digestion. Another possibility is that in ruminants, both storage and membrane lipids contain mostly saturated lipids, which have a higher melting point than unsaturated lipids. Laboratory experiments in which animals were fed diets rich in saturated or unsaturated lipids just before hibernation showed that, at least in small rodents, a larger proportion of unsaturated lipids in cell membranes and adipose tissue is essential to successful hibernation . Finally, pregnancy, which lasts a relatively long time in ruminants and usually takes place during the winter, could not be sustained at very low body temperatures.
