8 Water and its impurities
Water must be of a certain quality to be suitable for human consumption. No natural water found on Earth is pure; any sample of water contains more than just water molecules. Some materials, such as sodium nitrate, are very soluble and dissolve in water in large quantities, whereas other materials are much less soluble. This is just as well, otherwise rain would dissolve all the rocks and they would end up in the oceans!
Drinking water must not contain harmful materials, which means both harmful bacteria and dissolved material that could be dangerous. Water does not have to be absolutely pure to be drinkable and, indeed, not only would it be costly to make it so, but also water is an important source of many of the metal ions (such as Ca2+) required in the human diet. Water must be processed to make it acceptable for consumption. However, water must have levels of impurities that are below a danger threshold. How such thresholds (recommended by the World Health Organization) are assessed can be contentious but, in order to make comparisons, some measure of the amount of a particular substance dissolved in water is needed. Concentration is the mass of a substance in a known volume of a liquid: for example, milligrams in a litre. The units of concentration are expressed as milligrams per litre (abbreviated to mg/l).
Question 34
What fraction of a gram is a milligram?
Answer
A milligram is one-thousandth of a gram:
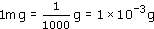
As noted above, tap water is never pure in the sense that it contains only water molecules and no other chemicals. Even bottled waters are not 100% water. Which substances are dissolved in water and in what concentrations?
If you drink bottled mineral water, look at the label and note down the contents. You will find that it contains a wide range of ions. Table 8 gives the concentrations of some ions in various bottled waters and two tap waters. Note that tap waters can vary substantially. You are already familiar with most of the ions listed.
| Ion | Concentration/(mg/l)* | |||||
|---|---|---|---|---|---|---|
| Volvic® | Vittel® | Buxton® | Evian® | Tap water | ||
| Area 1 | Area 2 | |||||
| calcium | 11.5 | 91.0 | 55.0 | 78.0 | 130.0 | 50.0 |
| magnesium | 8.0 | 19.9 | 19.0 | 24.0 | 9.4 | 6.6 |
| sodium | 11.6 | 7.3 | 24.0 | 5.0 | 51.0 | 128.0 |
| potassium | 6.2 | - | 1.0 | 1.0 | 9.4 | 1.6 |
| chloride | 13.5 | - | 37.0 | 4.5 | 82.0 | 27.1 |
| nitrate | 6.3 | 0.6 | <0.1 | 3.5 | 26.0 | 17.6 |
| sulfate | 8.1 | 105.0 | 13.0 | 10.0 | 210.0 | 21.3 |
| bicarbonate | 71.0 | 258.0 | 248.0 | 357.0 | ? | ? |
Footnotes
< means 'less than'; - means too small to measure; ? means the values are not available.Footnotes
*Note here that for clarity we have enclosed the units mg/l in brackets, i.e. the column heading is concentration divided by mg/l.Question 35
What are the formulas for the following ions: calcium, sodium, potassium, chloride and nitrate?
Answer
In Section 7 you saw that calcium is Ca2+, sodium is Na+, potassium is K+, chloride is Cl− and nitrate is NO3−.
To interpret Table 8, above, a few points need to be clarified. For example, what do the values in Table 8 mean? They are given as concentrations in mg/l.
Question 36
From Table 8 what is the concentration of chloride ions in Buxton water?
Answer
The concentration is 37 mg/1, which means that in one litre of water there are 37 mg of dissolved chloride ions.
You may be wondering why ion concentrations in drinking water are deemed important enough to be measured by suppliers of both bottled drinking water and tap water. The following activity will help to clarify this.
Activity 3: Ions in drinking water
The aim of this activity is to access information about ions in drinking water. Look at the information leaflets on the Drinking Water Inspectorate of England and Wales (DWI) website [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] (accessed 12 February 2016) and answer the questions below. (You will need to access information on water hardness and nitrate.)
- Which ions in Table 8 are responsible for the hardness of water?
- What is the World Health Organization’s guideline value for nitrate concentration in drinking water?
- How does this concentration compare with the values for nitrate concentration in Table 8?
When you accessed the DWI web pages on drinking water you may have noticed that it isn't just ion concentrations that are monitored. Water companies are also required to monitor their treatment works for the presence of Cryptosporidium (a micro-organism that can cause diarrhoea) and pesticides.
