3.7.2 Oxides
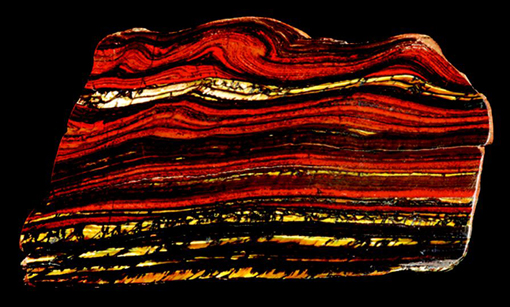
Oxides are an important group of non-silicate minerals. Hematite (Fe2O3) and magnetite (Fe3O4) are the two most common oxides of iron. Hematite contains iron in its most oxidised form (Fe3+) and often has a striking red-brown colour similar to rust (hydrated iron oxide) and is responsible for the intense red colour of some desert sandstones. Magnetite and hematite are found in banded iron formation deposits of Precambrian age which are the main sources of iron ore today (Figure 62). In most rocks, they are present only as accessory minerals.
Magnetite is a member of the spinel group of minerals, which have the general formula AB2O4, where A represents 2+ cations and B represents 3+ cations. A large number of elements can substitute into the spinel structure so that spinel group minerals are present in a wide range of igneous, metamorphic and sedimentary rocks. Chromite (FeCr2O4) is a chrome spinel found in silica-poor igneous rocks and is an important source of chromium.
-
Both hematite and magnetite are opaque minerals. How would you expect them to appear in plane-polarised light and between crossed polars?
-
Opaque minerals appear black both in plane-polarised light and between crossed polars, which makes specific identification difficult. By contrast, non-opaque isotropic minerals are black only when between crossed polars - which is an important distinction.
