4 The molecules involved
4.1 Salicylic acid
The structural formula of salicylic acid, 2.1, looks quite complicated. However, it becomes less daunting if you unpack it a bit. One of the first things to do when confronted with an unfamiliar structure is to check that all the valencies are correct (four for carbon, two for oxygen and one for hydrogen). If any atoms have the wrong valency, it follows that there is a mistake somewhere and the molecule does not exist as drawn. It looks OK for the structure of salicylic acid. You probably noticed that some of the carbon atoms have two bonds joining them to another atom. These are called double bonds and they contain four electrons, two per bond. They are quite common in chemical structures. There are two types of double bond in salicylic acid, carbon-carbon double bonds (C=C) and a carbon–oxygen double bond (C=O). Two of the thinner, more flexible bonds in the model kit are used when making models requiring a double bond in the structure. Make a model of each type of double bond in 2.1 and keep them for using later.
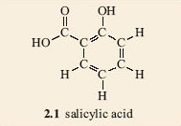
Let's have a closer look at the salicylic acid molecule. For a start, focus on the ring part of the structure. If the two groups attached to the ring (the side chains) are removed and replaced with hydrogen atoms, we are left with the hydrocarbon, benzene. This is a liquid, present in coal-tar, which used to be widely used as a solvent by chemists until it was discovered just how poisonous it is. Nowadays benzene is a product of the petrochemical industry.
Activity 1
1. Make a model of a benzene molecule, 2.2, with your model kit.
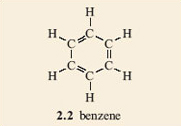
What do you notice about the model that makes it different to all the previous molecular models that you have made?
Answer
All the atoms lie in one plane; the ring structure is flat (planar). The presence of the double bonds is the cause of this.
2. Now turn your attention to the rest of the salicylic acid molecule. Remove the hydrogen from one of the benzene's carbons (it does not matter which one) and replace it with an oxygen joined to a hydrogen (an OH group). When this type of reaction is carried out for real, chemists refer to it as a substitution reaction. The structure that you have made is 2.3. You may need to rotate your model, or the —OH group on it, to match 2.3.
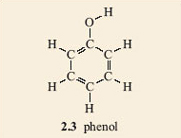
Note that the C—O—H sequence in 2.3 is not linear. Also the —O—H group in 2.3 can rotate freely around the C—O bond, giving many possible overall shapes for the molecule only one of which is planar.
The presence of the —OH group gives the molecule 2.3 particular properties that are not possessed by benzene. Such a group is called a functional group. The —OH functional group is called a phenol (pronounced fee-nol) group if it is joined to a benzene ring. The same word, phenol, is also used as the name of the compound you made (2.3) consisting of a benzene ring carrying an —OH group and no other substituent groups.
Activity 2
Now look at the other group on the benzene ring in salicylic acid, 2.1. Make a model of this group and substitute it for another hydrogen atom on your model of phenol. This time you will have to be a bit more particular about which hydrogen atom you substitute. It must be on a carbon atom that is adjacent to the phenol group. There is still some choice, though, as there are two of them.
Does it matter which of the two carbon atoms you change?
Answer
It appears that perhaps it does, as it looks as if there are two possible isomers. These are 2.4 and 2.5, and would certainly represent different molecules as they are not superimposable on each other. The difference is that 2.4 has a single bond between the substituted carbons and 2.5 has a double bond between these two carbon atoms. Notice how the structural formulae have been simplified. The benzene ring part of the molecule can just be shown as a hexagon with alternate double and single bonds. Chemists recognise that there is a carbon atom at each of the corners and each carbon atom must carry another group or atom to keep its valency correct. When no group is shown on a corner carbon atom, it is understood that a hydrogen atom is bonded to it. This style stops the drawn structures from getting too cluttered.
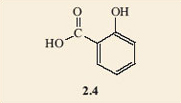
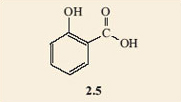
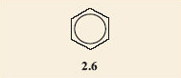
It is found that only one product results when the hydrogen atoms on two adjacent carbon atoms in a benzene ring are substituted by any two functional groups. All attempts to make two isomers end up producing the same product. This is an example of failure of the model to depict what the structures really are. In fact neither of structures 2.4 and 2.5 accurately depicts the structure of salicylic acid. A better model is a combination of both of them; you could regard it as a sort of average of the two structures, with one of the bonds in each double bond spread around the ring. This is often shown by drawing a circle in the ring, instead of three double bonds. The circle represents six electrons (two from each of the double bonds) shared round the ring. Structure 2.6 shows the structure of benzene drawn in this style and 2.7 depicts salicylic acid drawn in the same style.

Because the ball-and-stick models are unable to show these ‘spread-around-the-ring’ electrons, the structures for benzene rings where the double bonds are shown separately will be used. However, where necessary it should be remembered that these double bonds are shared round the ring. With this in mind, 2.4 and 2.5 turn out to be representations of the same molecule, within the limitations of what the ball-and-stick models can show.
‘Spread-around-the-ring’ or ‘delocalised’ electrons do confer some special properties to benzene and similar compounds. Benzene is said to be ‘aromatic’ or to have ‘aromatic properties’. Although the word aromatic originated because many of these compounds had a characteristic odour it no longer means anything to do with smell – in fact some aromatic compounds have awful smells. It now just means a ring structure with delocalised electrons.
Note that the —COOH group that you have put on the benzene ring contains an —OH group. However, the close proximity of the C=O part of the molecule changes the properties of the —OH group. In fact the whole of the group (one carbon, two oxygens and a hydrogen atom) is named as one functional group. It is a carboxylic acid group and has a whole set of chemical properties that make it different to the —OH group. To simplify writing this functional group it is often abbreviated to —COOH or —CO2H. Some people refer to it as a ‘coo’ (rhymes with blue) group in conversation.