5 Some chemistry involving esters
Esters are produced by the reaction of a carboxylic acid with an alcohol and result from the formation of a new bond (Reaction 2.1). For example, ethyl butanoate, the major constituent of artificial pineapple flavouring, is made from the reaction of butanoic acid with ethanol.
![]()
There is a certain logic to the naming of these compounds. Note the endings on the names: -ic for the carboxylic acid, -ol for the alcohol and -ate for the ester.
Because the other product is water, this type of reaction is known as a condensation reaction. (There are other condensation reactions that do not involve the formation of water, but they do involve two molecules joining together to form a larger molecule with the elimination of a different smaller molecule, e.g. ammonia or hydrogen chloride, instead of water. We will not be concerned with these other condensation reactions.) This condensation reaction is common to nearly all carboxylic acids, R1COOH, and alcohols, R2OH. So, we can write the general reaction, 2.2, where the abbreviation R1 represents the rest of the carboxylic acid molecule and R2 represents the rest of the alcohol molecule. Check through Reactions 2.1 and 2.2 and make sure you can follow the way in which some of the atoms move from one molecule to another as the reaction takes place. We have colour coded the reacting groups in Reactions 2.1 and 2.2 to help you see this change.

The use of the symbol R to represent the rest of the molecule is common in organic chemistry. It enables us to focus on the parts of the molecules that matter (the functional groups) without the formulae being cluttered up with the parts that are not reacting.
Question 3
Which carboxylic acid and alcohol would you use to make isopentyl acetate, 2.9, a constituent of banana oil? (Hint: you need to identify R1 and R2.)

Answer
Reaction 2.3 shows the required carboxylic acid and alcohol.
![]()
An important thing about this reaction is that, although it shows an ester forming from a carboxylic acid and an alcohol, it could equally well have been written the other way round. It would then show the reaction of an ester with water, to produce a carboxylic acid and an alcohol. This is an example of a chemical reaction that can run in either direction, rather unsurprisingly known as a reversible reaction. They are very common and sometimes you see the use of the symbol ![]() instead of =. The reverse of the condensation reaction above is known as a hydrolysis reaction, reflecting the fact that it is reaction with water. The word is derived from hydro (water) and lysis (splitting), so hydrolysis is literally splitting with water, which accounts for the fact that any hydrolysis reaction gives two products.
instead of =. The reverse of the condensation reaction above is known as a hydrolysis reaction, reflecting the fact that it is reaction with water. The word is derived from hydro (water) and lysis (splitting), so hydrolysis is literally splitting with water, which accounts for the fact that any hydrolysis reaction gives two products.
Question 4
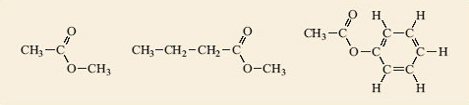
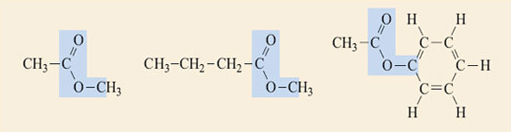
Identify the ester groups in the compounds shown in Figure 2. Write down the reactions that produce each of the esters and thus deduce the structures of the carboxylic acids and alcohols needed to make them.
Now look back to the structures of salicylic acid, 2.7, and aspirin, 2.8.
Question 5
See if you can pick out the carboxylic acid that is involved in converting the phenol group in salicylic acid into the ester group in aspirin.
Answer
You should have decided it was CH3—COOH, acetic acid, but may well have written its formula in a different style or shape. This does not matter, it is still acetic acid so long as the atoms are joined together in the same order as in our structure,
Activity 3
Complete your study of the relationship between salicylic acid, acetic acid and aspirin by making models of salicylic acid, 2.7, and acetic acid. Place them side by side so that the phenol group (—OH) of the salicylic acid is next to the —COOH of the acetic acid. Then remove the H atom from the phenol and the —OH from the acetic acid and join the two fragments together. You have made an aspirin molecule, 2.8. What else have you made?
The other compound that you have made is water, H2O (remember this is a condensation reaction), provided you joined the unwanted H atom to the —OH. The complete reaction is shown in Figure 4.
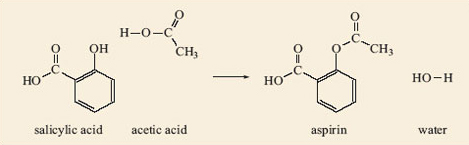
You should be able to see how the reaction in Figure 4 can take place in the reverse direction (hydrolysis), with the water molecule splitting the aspirin molecule into molecules of salicylic acid and acetic acid. If you have an old bottle of aspirin tablets you may find they have a slight smell of vinegar, especially if they have become damp. As you have seen, when aspirin reacts with water the carboxylic acid that it forms, by hydrolysis of the ester group, is acetic acid. This is the compound responsible for the smell of vinegar.