8 Enter aspirin!
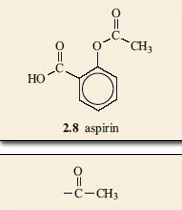
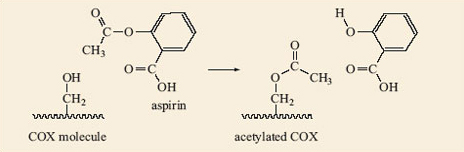
Aspirin is able to release part of its ester group (Figure 15) in a hydrolysis reaction. Look again at the structure of aspirin, 2.8, and identify this group on the molecule. It is known as an acetyl group and accounts for aspirin also being called acetylsalicylic acid. The acetyl group on aspirin is fairly easily removed and can be available for forming another ester with an —OH group on another molecule; in this case, part of the structure that makes up the inside of the cavity in COX (Figure 16). The wiggly line in this reaction represents the rest of the COX molecule.
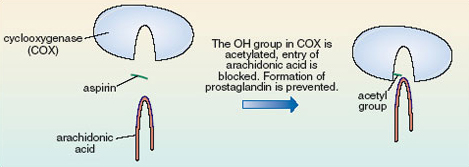
The —OH group in the COX cavity has become acetylated, i.e. had an acetyl group added to it. Quite simply this just makes the cavity of the active site of COX smaller. The arachidonic acid is no longer able to enter the cavity, so prostaglandin does not form, so the pain is relieved (Figure 17). This acetylation involves the formation of a covalent bond which is strong, so the acetyl group is not readily released and aspirin continues to relieve pain for quite a long time after the dose is taken.
Question 6
Write a list of the main events in ‘the story of aspirin’, from the time of Hippocrates to when Bayer started marketing aspirin.
Answer
The steps in the development of aspirin are shown below. There are likely to be other perfectly acceptable answers that show slight variations.
| Step 1 | Willow bark and leaves known to be effective painkillers for thousands of years. Salicin extracted from willow bark during the 1840s. |
| Step 2 | Salicin (lead compound) tested and found to be the active ingredient in willow bark and leaves. |
| Step 3 | Structure of salicin investigated. |
| Step 4 | Salicylic acid synthesised. |
| Step 5 | Salicylic acid found to be effective but irritant. |
| Step 6 | Sodium salicylate made. Effective for pain relief, not irritant but tasted awful. |
| Step 7 | Synthesis of other compounds related to salicylic acid. |
| Step 8 | Hofmann tested them (on his father). |
| Step 9 | Aspirin emerged as the suitable compound. |
Question 7
What is the molecular formula of aspirin?
Answer
The molecular formula just tells you how many atoms of each type there are in one molecule of the compound, so for aspirin it is C9H8O4.
(There are six C atoms in the ring plus three in the side-chains, giving nine overall. There are four H atoms attached to the ring carbons that do not carry side-chains plus four H atoms in the side-chains, so a total of eight. There are two O atoms in each side-chain, making four in all.)
Question 8
Make a model of a molecule of cyclohexane, a hydrocarbon with molecular formula C6H12 that contains a six-membered ring of carbon atoms. Compare it to a model of benzene and comment on their different shapes.
Answer
You should have found that all the atoms in the benzene molecule lie in one plane, but this is not so with cyclohexane. This is because cyclohexane does not have any double bonds to restrict the shape that the molecule can adopt.
Question 9
Each cell in the human body accommodates around 2000 different enzymes but only a small quantity of each one. Why are there so many and why is only a small quantity of each required?
Answer
Enzymes are highly specific in their action. That is to say each enzyme will only catalyse a very small range of chemical reactions, or perhaps only one. As a human cell needs to carry out a very great number of different reactions it needs a wide range of different enzymes. It only needs a small amount of each enzyme because enzymes are not used up or converted into different chemicals when they perform their function of catalysing cell reactions, so are recycled.