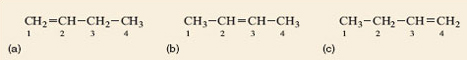6 How does aspirin relieve pain?
Aspirin acts at the site of damaged tissue to block the start of the nerve signal to the brain, the mechanism by which we experience pain (Section 2). It does this by inhibiting the formation of prostaglandin which is the active agent responsible for the sensitisation of the nerve endings. Prostaglandins (there are several related types) are responsible for a lot of physiological events in addition to the start of the pain signal at the nerve ending. For example, they are responsible for inflammation, fever and the clotting of platelets in the blood, so it is easy to see why aspirin is such a useful drug. However, a prostaglandin also increases the formation of protective mucus in the gut, so aspirin-induced suppression of its formation can cause irritation and bleeding.
Before an explanation of aspirin's activity at the molecular level can be developed some more chemical ideas are needed. Prostaglandins are synthesised in the body's cells from arachidonic acid, 2.10. This is a carboxylic acid containing a chain of 20 carbon atoms in its molecule. 2.10 shows this structure.
![]()
Although this structure looks rather complicated at first, it is relatively simple in that it has an unbranched carbon chain terminating in a carboxylic acid group. It does have the added complication of four carbon–carbon double bonds but the real influence of these is not apparent until one looks at the shape of the molecule. We have made a model of 2.10 and photographed it, but before we look at that you need to return to your model kit and have a look at the shape surrounding the atoms joined by a carbon-carbon double bond.
Activity 4
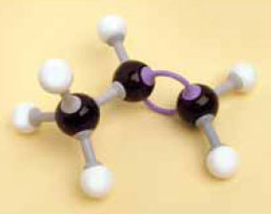
1. Make a model of the compound ethene, C2H4, using the appropriate number of bonds and keeping the valencies correct for all the atoms.
You should have found that you needed a double bond between the two carbon atoms (remember to use two of the longer bonds in the kit for this) and should have ended up with the structure shown in Figure 5.
If you think back to previous models you made, you should recall ethane, CH3—CH3, the second member of the homologous series known as the alkanes. Note the similarity in the names. The ending –ane has been changed to –ene, but the rest of the name (eth-) is preserved because it refers to the fact that there are two carbon atoms in the molecule. Ethene is the simplest member of the homologous series known as the alkenes. The simplest alkane, methane, does not have a counterpart in the alkene series, as it is not possible to have a double bond if there is only one carbon atom in the molecule. After this, though, the alkenes follow a similar naming sequence as the alkanes, so you have propene, butene and so on.
2. Now take your model of ethene. Remove one of the hydrogen atoms, extend the carbon chain by one carbon atom and add the appropriate number of hydrogen atoms to keep all the valencies correct. You now have a model of a propene molecule.
This should have been straightforward enough and our model is depicted in Figure 6.
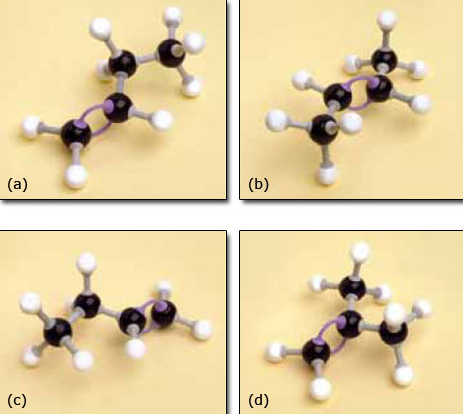
3. Now take your model of propene and extend it by one carbon atom plus the appropriate number of hydrogen atoms to make a molecule of C4H8.
You probably found this less straightforward as you could join the extra carbon atom to any one of the three carbon atoms in propene and it looks as if you end up with several different molecules.
The molecules are shown in Figure 7. All these molecules exist and we need a way of naming them that makes it clear which molecule is being discussed.
The structure shown in Figure 7d does not present a problem. It has a methyl group attached to an alkene with three carbon atoms in the chain and so it is named methylpropene.
Before attempting to name the remaining structures, Figure 7a–c, we need a way to identify exactly which carbon atoms are joined to the double bond. This is done by numbering the chain, starting at one end, as shown in Figure 8.
As Figure 7a and b cannot be exactly superimposed on each other they are molecules of different compounds. They are named but-1-ene and but-2-ene, respectively, the number in the name designating the lowest numbered carbon atom in the chain to which the double bond is joined. It might be expected that Figure 7c is but-3-ene but this is not the case because it is identical to but-1-ene (Figure 7a). It can be picked up, turned over and superimposed on but-1-ene, so is the same – try it with your models. By convention the lowest possible number(s) is always used in a name whenever there is more than one option. Because but-1-ene, but-2-ene and methylpropene have the same molecular formula (C4H8) but different structural formulae (showing which atoms are joined to which) they are isomers (Section 1.4).
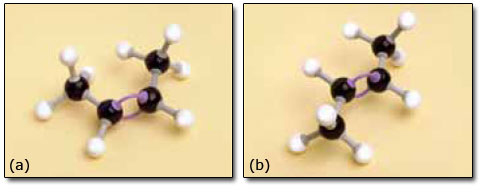
You might have found that but-2-ene created a problem in that you needed to decide which side of the double bond to place the two —CH3 groups and the two hydrogen atoms. Our models are shown in Figure 9.
The problem was caused by an important feature of the model that is also possessed by the molecule itself. Two carbon atoms joined by a double bond cannot rotate relative to each other. This part of the molecule is therefore much more rigid than parts built with just single bonds. Cis-but-2-ene and trans-but-2-ene have the same structural formula and the same sequence of atoms is present in both. However, their geometry differs so one cannot be superimposed on the other, so they really are different compounds. They are known as geometrical isomers, or cis/trans isomers (Latin: cis, on this side; trans, across).
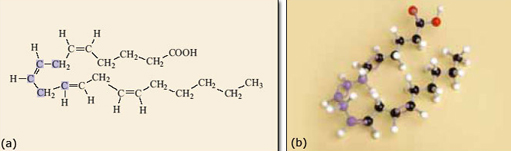
Returning to the structural formula of arachidonic acid, introduced earlier, the implications soon become apparent. The double bonds control the shape of the molecule.
![]()
There are four double bonds in the molecule, each of which can be cis or trans, so generating a pair of geometrical isomers. If all the different geometrical isomers of 2.10 are modelled that makes a total of 16 different possibilities in all! However, because double bonds prevent rotation within the molecule only one of the 16 isomers has the atoms in the correct place to form prostaglandin. The relevant isomer is the one where all the double bonds are cis, as shown in Figure 10. This isomer needs to react with oxygen and form the ring near the middle of the carbon chain (Figure 11). If any of the double bonds are trans, the atoms are too far apart to react to form the five-membered ring and the structure shown in Figure 11 could not form.
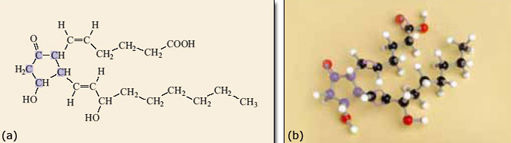
The formation of a five-membered ring in the arachidonic acid molecule to produce prostaglandin could not take place in the body without the action of enzyme molecules. These are compounds that increase the rates of reactions in living systems.