Transmission electron microscopy
Transmission electron microscopy has been greatly refined since the first commercial electron microscope became available in 1939. TEM has allowed detailed examination of cell ultrastructure and assisted the identification and investigation of cell organelles such as the Golgi apparatus (Section 4.7), which had previously been seen only as indistinct subcellular structures using histochemical techniques and light microscopy.
As in light microscopy, samples must be fixed and processed before they can be viewed using a transmission electron microscope (Figure 2a) although the reagents used are different from those used for light microscopy. Preservation of the tissue is very important in this technique; it is crucial that the membranes of the cell organelles are preserved and not obscured by precipitates (insoluble deposits) formed during fixation. Glutaraldehyde, which fixes proteins, followed by osmium tetroxide, which fixes lipids, are the fixatives most often used. The fixed tissue is embedded in a very rigid resin, such as epoxy resin, which allows very thin sections to be cut on an ultramicrotome. 'Ultrathin' EM sections, which are used for study of cell organelles, are typically around 70 nm thick (compared with the 5-50 µm thick sections typically used for light microscopy). The image is obtained by passing electrons through the section, and focusing them on a fluorescent screen that emits visible light where electrons strike it. The interior of the microscope is under vacuum (to prevent scattering of the electron beam by air molecules) and the direction of the electron beam is controlled by magnets.
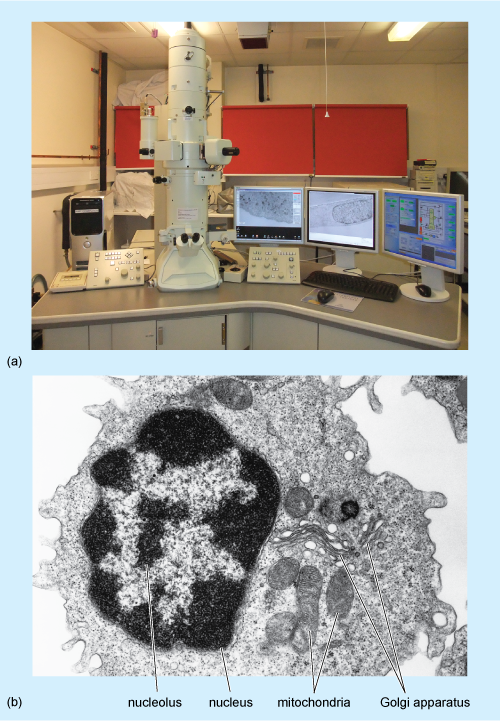
Electrons pass readily through unstained tissue sections, because the cell components are made up of small atoms such as carbon, oxygen and hydrogen. To enable cellular components such as organelles to be viewed, the sections must first be 'stained' to increase contrast. However, unlike light microscopy, which uses coloured stains that absorb light, in TEM, the stains contain heavy metals, such as lead and uranium. These are large atoms that prevent electrons passing straight through the section. Uranium binds preferentially to nucleic acids and proteins, while lead binds preferentially to lipids. So, after staining, cell components that are rich in lipids and areas where proteins and DNA are concentrated prevent the passage of electrons and so appear relatively dark, or 'electron-dense', on the viewing screen. Areas in which proteins are less concentrated, such as the cytosol of animal cells, appear pale, or 'electron-lucent'. A typical TEM image, of a white blood cell, is shown in Figure 2b.