1.2.2 Bringing in the atmosphere: the natural greenhouse effect
As a dam built across a river causes a local deepening of the stream, so our atmosphere, thrown as a barrier across the terrestrial rays, produces a local heightening of the temperature at the Earth's surface.
(Tyndall, 1862, quoted in Weart, 2004)
Thus, writing in 1862, John Tyndall (Figure 6) described the key to our modern understanding of why the Earth's surface is so much warmer than the effective radiating temperature. Tyndall's careful experimental work had established what others only suspected: expressed in modern scientific terms, certain atmospheric gases absorb infrared radiation with wavelengths in the range spanned by outgoing terrestrial radiation (about 4 to 100 μm; Figure 5). These are the greenhouse gases. Tyndall identified water vapour and CO2, but the list of natural greenhouse gases (naturally present in the atmosphere long before human activities began to make their mark) also includes methane (CH4), nitrous oxide (N2O) and ozone (O3). The main mechanism by which these gases absorb infrared radiation is through the vibrations of their molecules. We shall not pursue the scientific principles that underlie this mechanism in any detail, but the key points we shall need are summarised in Box 2.

Like many Victorian scientists, Tyndall was interested in a great many questions - contributing to such diverse areas as heat transfer, glacier motion and scattering of light in the atmosphere, where he is honoured for his explanation of why the sky is blue (the Tyndall effect). He was a keen alpinist, and attracted by one of the great riddles of his day: if vast sheets of ice had once covered all of northern Europe (hotly debated at the time), how could climate have changed so radically? One then-current hypothesis was a change in atmospheric composition, and it was this possibility that led to Tyndall's pioneering work on the physics of the greenhouse effect. He was also a committed communicator; during his time at the Royal Institution, he earned great renown for presenting science to the public. So it is fitting that one of the climate change research institutes in the UK, with a particular focus on an interdisciplinary approach and communication with the public, local authorities, business, etc., is named after him - the Tyndall Centre in Norwich.
Box 2 'Exciting' molecular vibrations
The chemical bonds that hold a molecule together are like springs and, like them, they can stretch and flex, making the molecule vibrate. Molecular vibrations always have a characteristic frequency. If a molecule absorbs radiation of a matching frequency - and hence with a characteristic wavelength (see Box 1) - the energy it gains makes it vibrate more vigorously. The frequencies of molecular vibrations invariably correspond to wavelengths in the infrared part of the spectrum.
To be 'infrared active' (i.e. to absorb infrared radiation through changes in the way it vibrates), a molecule must contain more than two atoms or, if there are just two atoms, these are of different elements. More complex molecules, such as the greenhouse gases, can vibrate in several ways, each with its own characteristic frequency. So they can absorb a range of wavelengths in the infrared.
Once 'excited' by absorbing infrared radiation, a greenhouse gas molecule can lose energy again by re-emitting radiation of the same wavelength. Alternatively, it can pass energy on to other molecules in the air by bumping into them: the net effect is to increase the total 'energy content' of the air, warming it up.
Taken together, the natural greenhouse gases absorb infrared wavelengths throughout most of the terrestrial range; there is only one region, between 8 and 13 μm, where absorption is weak. Known as the 'atmospheric window', this allows some of the longwave radiation from the surface to escape directly to space, but most of it is intercepted by the atmosphere. That changes the simple picture in Figure 4 substantially. A better representation is shown in Figure 7. Now most of the longwave radiation from the surface is effectively 'trapped' and recycled by the atmosphere, being repeatedly absorbed and re-emitted in all directions by the greenhouse gases. This warms the atmosphere. Some of the re-emitted radiation ultimately goes out to space, maintaining an overall radiation balance at the top of the atmosphere, as shown in Figure 7. This prevents the whole Earth-atmosphere system from heating up without limit. The crucial difference is that much of the re-emitted radiation goes back down and is absorbed by the surface. It is this additional energy input - over and above the absorbed solar radiation - that keeps the Earth's GMST over 30 °C warmer than it otherwise would be.
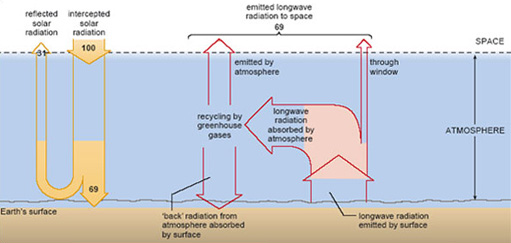
As in Figure 4, Figure 7 shows that 69 units of solar radiation are absorbed by the planet and 69 units of longwave radiation go back out to space. However, this overall radiation balance is now at the top of the atmosphere, not at the surface, which receives an extra input of energy through the 'back radiation' from the atmosphere.
The surface warming attributed to the back radiation from the atmosphere is called the greenhouse effect.
The contribution each of the greenhouse gases makes to the total effect depends on two main factors: how efficient it is at absorbing outgoing longwave radiation, and its atmospheric concentration. The striking thing is that most of these gases are only minor atmospheric constituents, as shown by the information collected in Table 1. Here, concentrations are given as 'mixing ratios' - the measure of atmospheric composition that has become familiar to policy makers and other stakeholders in the climate change debate (Figure 8). The term is explained in Box 3.

Box 3 Mixing ratios
Strictly, the mixing ratio (by volume) tells us about the 'fractional abundance' or proportion of a given atmospheric gas, although you will often find it referred to as the 'atmospheric concentration' (and we shall follow this practice). Taking oxygen (O2) as an example, the formal definition is as follows:
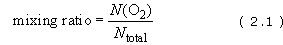
where N total is the total number of molecules in a given volume of air (a cubic metre, say) and N(O2) is the number of molecules of oxygen in the same volume of air. Expressing the fraction in decimal form or as a percentage (by multiplying by 100) is fine for the major atmospheric constituents (see the entries in Table 1), but it becomes unwieldy for minor constituents like the greenhouse gases. In this case, values are usually recorded as ppm (parts per million, 106) or as ppb (parts per billion, 109) - or even as ppt (parts per trillion, 1012) for the least abundant species.
SAQ 5
In Table 1, the mixing ratio of CO2 is given as 368 ppm. Express this value as a number (in scientific notation), and then as a percentage.
Answer
A value of 368 ppm means that in every million molecules of air, 368 will, on average, be molecules of CO2. So 368 ppm is equivalent to 368/106=368 × 10−6=3.68 × 10−4 (in scientific notation). Multiplying by 100, this becomes 3.68 × 10−2% or 0.0368%.
SAQ 6
Now express the mixing ratio of CO2 in ppb.
Answer
If there are 368 molecules of CO2 per million in total, there would be 368 000 per billion, so the answer is 368 000 ppb.
Thus, 1 ppm=103 ppb, and similarly 1 ppb=103 ppt.
| Gas (and formula) | Mixing ratio |
|---|---|
| major constituents | |
| nitrogen (N2) | 0.78 |
| oxygen (O2) | 0.21 |
| argon (Ar) | 0.0093 |
| trace gases | |
| carbon dioxide (CO2) | 368 ppm |
| methane (CH4) | 1745 ppb |
| nitrous oxide (N2O) | 314 ppb |
| ozone (O3) | 10-100 ppb |
SAQ 7
Given the information in Table 1, how would you describe the bulk composition of the lower atmosphere?
Answer
99% is nitrogen and oxygen (roughly in a 4 : 1 ratio), and most of the rest (0.93%) is argon.
SAQ 8
Is any one of these major components a greenhouse gas?
Answer
No. The chemically inert noble gas argon exists as individual atoms; nitrogen and oxygen molecules each consist of two atoms of the same element. None of them fulfils the criterion for being infrared-active (Box 2).
Note that the mixing ratios in Table 1 are for dry air. The contribution from water vapour is not included because the amount in the air is highly variable - from practically none at all up to about 4% (by volume). Part of the explanation is that air can 'hold' only a certain amount of water vapour: it has a 'saturation' limit, which depends mainly on temperature. The variable humidity of the air (a measure of its water vapour content) is part of our everyday experience: it affects the ability of sweat to evaporate, for example, and the drying of clothes on the line.
Averaged over time and around the globe, water vapour represents about 0.5% of the total atmospheric gas. This relatively high abundance makes water vapour the single most important natural greenhouse gas: it contributes about 60% of the surface warming attributed to the natural greenhouse effect. Carbon dioxide, the second most abundant, contributes a further 25% or so; most of the rest is due to the other three trace gases in Table 1, which have much lower atmospheric concentrations. (One further contribution is noted in Section 1.3.3.)
The fact that the Earth is not a frozen and lifeless rock shows that the natural greenhouse effect is not a 'bad thing'; indeed, it is a 'good thing'! It is the extra warming produced by an enhanced or amplified greenhouse effect, due to an increase in the atmospheric concentration of CO2 (and indeed other greenhouse gases), that lies at the heart of current concerns. We shall sometimes refer to this as an increase in the atmospheric 'burden' of CO2 (or of greenhouse gases in general), since an increase in concentration necessarily implies an increase in the total amount (or number of molecules) of the gas in the atmosphere.
Question 2
Analogies are a useful aid to understanding, and can be a powerful means of communicating scientific ideas to a lay audience. However, they can be misleading. Look back at the quote from John Tyndall at the beginning of Section 1.2.2. In what way is the analogy used there a misleading one? Explain your reservations, making reference to the mechanism that actually creates the Earth's greenhouse effect.
Answer
The basic problem is the notion of a 'barrier across the terrestrial rays'. This could suggest that the atmosphere somehow 'reflects' back outgoing radiation (an error that sometimes appears in newspaper accounts to this day) and/or that none of it ever goes out to space - in which case the planet would simply heat up without limit! In reality, some of the longwave radiation from the surface escapes directly to space (at wavelengths in the 'atmospheric window'). The rest is absorbed and re-emitted (up and down) by greenhouse gases in the atmosphere. Back radiation from the atmosphere keeps the surface warmer than it otherwise would be (the natural greenhouse effect). But some of the re-emitted radiation ultimately goes out to space, maintaining an overall radiation balance at the top of the atmosphere.
