1.3.1 The vertical 'structure' of the atmosphere
The atmosphere is not a simple, uniform slab of absorbing material. On the contrary, it gets progressively 'thinner' or less dense with increasing altitude (height above mean sea level); i.e. the total number of molecules in a given volume of air is lower, and so is the pressure. About 80% of the total mass of the atmosphere is within some 10 km of the surface; 99.9% lies below 50 km.
The important corollary is that the key greenhouse gas molecules (H2O and CO2) are also more abundant close to ground level, and increasingly scarce at higher altitudes. So a better picture of radiation trapping in the real atmosphere is to imagine it happening in a series of stages. Outgoing longwave radiation is repeatedly absorbed and re-emitted as it 'works up' through the atmosphere; it is re-radiated to space only from levels high enough (i.e. thin enough) for absorption to have become weak. This suggests that the atmosphere should be warmer at ground level - close to the source of the outgoing radiation, and where the absorbing molecules are more abundant. Everyday experience confirms this expectation; it generally gets colder as you walk up a mountain, for example.
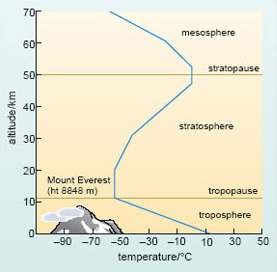
Figure 9 is a typical temperature profile of the atmosphere. It shows that air temperature does indeed fall with increasing altitude throughout the lower atmosphere or troposphere, reaching a minimum value (of about −55 °C) at the tropopause. This lies 8-15 km above the ground, depending mainly on latitude: it is higher (and colder) at the Equator than at the poles. No mountains rise above the troposphere; it is where we live and where almost all weather phenomena (rain, clouds, winds, etc.) occur. However, if you could travel higher up (without the protection of a jet aircraft), you would find that the temperature soon starts to increase again - and continues to do so up to the stratopause at the top of the stratosphere. Why is this?