3.6.2 Feldspars
Feldspars are especially common minerals and make up about 60% of the Earth's crust. They crystallise from a wide spectrum of magmas and are found in many metamorphic and sedimentary rocks. The name 'feldspar' refers to a group of silicate minerals that share the same basic structure. The three most important feldspar minerals are orthoclase (KAlSi3O8), albite (NaAlSi3O8) and anorthite (CaAl2Si2O8).
At high temperatures there is complete solid solution between orthoclase and albite, as K and Na substitute for each other (in the same way that Fe and Mg substitute for each other in olivine). Feldspars with compositions within this range are called alkali feldspars. At lower temperatures the alkali feldspar solid solution is not complete, and if an alkali feldspar crystal of an intermediate composition (e.g. Na0.3K0.7AlSi3O8) were cooled very slowly, it would 'unmix' into Na-rich and K-rich phases - a process known as exsolution (meaning 'from solution'). The result is a crystal mainly of one composition (K-rich), containing streaks of another composition (Na-rich), producing what is known as perthitic texture (particularly noticeable in thin section when viewed between crossed polars). You will see examples of perthitic texture in Figure 54 and Activity 3.7.
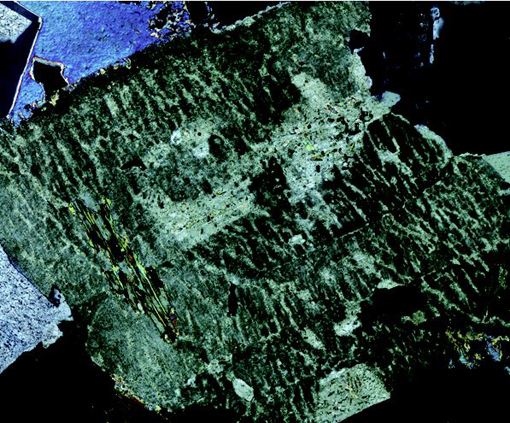
There is also complete solid solution between albite and anorthite at high and intermediate temperatures: these are the plagioclase feldspars. Plagioclase feldspars crystallise from a range of magma compositions, depending on the proportion of Ca and Na available. Solid solution in plagioclase feldspars is rather more complex than in alkali feldspars, because Na+ and Ca2+ have different charges. A coupled substitution is required to maintain charge balance whereby two substitutions occur simultaneously:
Na+ substitutes for Ca2+and Si4+ substitutes for Al3+
This coupled substitution is more usually written as:
Na+ + Si4+![]() Ca2+ + Al3+
Ca2+ + Al3+
There is no solid solution between KAlSi3O8 and CaAl2Si2O8 because of the large size difference between K+ and Ca2+ (about 30%). Ionic substitution is, therefore, not possible.
The overall compositional range of feldspars can be shown on a ternary diagram (Figure 55). Ternary diagrams are very useful tools in geology, and you will meet them again later in the course. Their use is explained in Box 4.
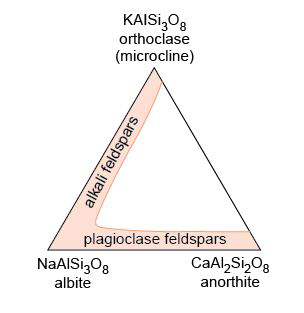
Box 4 Ternary diagrams
A ternary diagram can be used to plot the composition of a mineral (or indeed, any multi-component substance or mixture) in terms of three end-member components (A, B and C). The diagram consists of a triangle, with each corner representing one of the three end members (100% A, 100% B or 100% C) (Figure 56a).
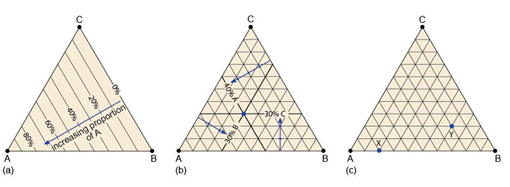
To plot a mineral's composition, you need to represent the proportion of each component. In Figure 56a, different proportions of component A are represented by lines parallel to the edge BC (0% A). Likewise, different proportions of component B would be represented by lines parallel to the edge CA (0% B), and so on.
To plot a mineral whose composition is: 40% A, 30% B and 30% C, you:
- determine on which 'A' line the 40% composition must lie (as in Figure 56a and b)
- find the 30% 'B' line (Figure 56b).
The intersection of these two lines at a point marks the composition of the mineral.
As a final check, you can identify the 'C' line, which should pass through the same point (Figure 56b).
SAQ 8
Using the ABC ternary diagram in Figure 56c, determine the compositions indicated by the two points X and Y.
Twinning in feldspar
You were introduced to twinning in Section 1.5. The presence of twinning is a common feature of feldspars and can be a useful diagnostic property.
-
Look at the twinned orthoclase feldspar in Figure 57a. With reference to the schematic above the specimen, you should be able to see that the crystal is twinned. How are the two twin components related?
-
The crystal appears to be made of two parts, which look very similar when viewed from different directions - and yet they seem 'squashed' together. These are twin components that overlap with each other (they are said to be inter-penetrant) and are related by a 180° rotation along the long (z) axis of the crystal (Figure 57a).
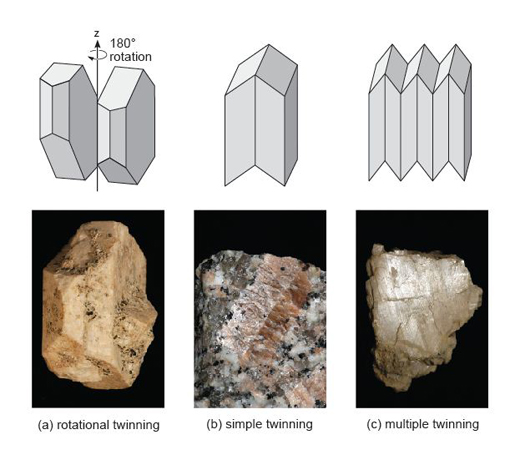
This crystal is a kind of growth twin, called a Carlsbad twin, which is especially common in K-feldspar. The two twin domains are related by a 180° rotation (Figure 57a). Twinning in feldspars does not have to be inter-penetrant and other types of twinning may occur by reflection along twin planes, producing either simple or multiple twinning (Figure 57b and c).
How might the feldspars be distinguished? In plane-polarised light, feldspars have low relief and are colourless. Both alkali and plagioclase feldspars are susceptible (though not necessarily under the same conditions) to alteration to fine-grained micas and clay minerals, which often gives them a turbid (dirty-looking) appearance (e.g. Figure 58b). However, both plagioclase and alkali feldspars have some distinctive features under the microscope.
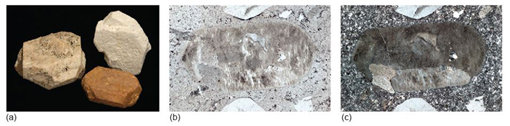
-
Would you expect to be able to see simple twinning in a thin section of a feldspar when viewed with plane-polarised light?
-
Not normally. There is unlikely to be a significant difference in colour or relief between the two twin components.
-
What would happen if the section were viewed between crossed polars?
-
On either side of the twin boundary, the crystal orientations would be different. Therefore the adjacent twin domains would go into extinction at different times. As the microscope stage was rotated, first one half of the twin would go into extinction, then the other half (e.g. Figure 58c).
Twinning in feldspars is most obvious between crossed polars. In addition to simple twins, plagioclase feldspar often shows repeated or multiple twinning (Figures 57c and 59a) usually on a microscopic scale, which gives crystals a striped appearance (Figure 59c). This is often referred to as lamellar twinning and is a characteristic feature of plagioclase feldspars.

One variety of K-feldspar, known as microcline, shows two kinds of repeated twinning in thin section, with one set of twins arranged at 90° to the other set. The lamellar twins overlap each other and have 'fuzzy' edges, giving a 'tartan' appearance known as cross-hatched twinning (see Figure 60).

Activity 3.6 Simple twinning in orthoclase feldspar
This activity will help you to recognise orthoclase feldspar in hand specimen and thin section.
For this activity you will need the Digital Kit [Tip: hold Ctrl and click a link to open it in a new tab. (Hide tip)] and the Virtual Microscope.
- The orthoclase mineral samples shown in the Digital Kit (see Feldspar (orthoclase) - 'Twinned crystals') show interpenetrant twins. The rotating specimen in the movie is particularly useful to gain a better understanding of the manner in which the crystals have intergrown.
- The pink phenocrysts of orthoclase feldspar are particularly striking in the granite rock specimen of the Digital Kit. Go to the orthoclase crystal granite in the Digital Kit ('Phenocryst in granite') and look at the broken surface. Can you see any flat, reflective surfaces? These are cleavage surfaces. In the Digital Kit, a reflective cleavage surface is visible in the matching image ('Twinning in phenocryst'). Cleavage directions differ on each side of the twin plane that runs down the middle of the crystal. This is a simple twin.
- Now look at the full-section view of the porphyritic rhyolite thin section using the Virtual Microscope (found under the ‘Igneous rocks’ category). In thin section you should see some large, cloudy, or turbid, crystals. These are orthoclase feldspar. Feldspars are often turbid like this, because of alteration - this provides a way of distinguishing feldspars from quartz, which forms the large, clear, colourless crystals, a few of which have faces at 120° in this thin section. Look under low power at the field of view centred at coordinates 3800,1300, using plane-polarised light, so that you can see the difference between the clear, low-relief quartz, and the turbid feldspar crystals.
- Now observe this view between crossed polars. Note that some of the feldspar crystals appear to be divided into two parts. In View 1, at the very edge of the field of view between crossed polars, you can see there is a straight-line division between two parts of the feldspar crystal that go into extinction in different positions. This is a twin plane, and the crystal is a simple twin; the two parts are in slightly different orientations to each other (Figure 58c).
Activity 3.7 Multiple twinning in feldspar
This activity will help you to recognise plagioclase and microcline feldspars in thin section. For this activity you will need to view plagioclase feldspar in the Digital Kit and the diorite and granite thin sections using the Virtual Microscope (both of which can be found under the ‘Igneous rocks’ category).
- Examine the rotation video clip of plagioclase feldspar in the Digital Kit and note that reflecting parallel stripes become visible briefly as the crystal is rotated (at about 80-95°). These reflections are the result of multiple twinning. This effect is not very commonly seen in hand specimen, but is very common in thin section as demonstrated below.
- Examine the diorite thin section at low magnification between crossed polars. You should see that a lot of crystals show regular dark and light stripes (like a 'zebra' crossing). These crystals are plagioclase feldspar, and they contain multiple twins - with twin planes developed in only one direction. This kind of twinning is lamellar twinning. Lamellar twinning (Figure 59c) is characteristic of plagioclase feldspar, and provides a good way of identifying this mineral in thin section.
- Now examine the granite thin section. You should find a mineral that shows a rather diffuse grid-like pattern when viewed between crossed polars (View 2). This mineral is a type of K-feldspar called microcline, distinctively showing two sets of multiple twins, developed at right angles to each other. This is called cross-hatched (or 'tartan') twinning (Figure 60).
Activity 3.8 Zoning in plagioclase
This activity will help you to recognise compositional zoning of crystals as developed in plagioclase feldspar. For this activity you will need to view the granite thin section using the Virtual Microscope.
The extinction angle of plagioclase varies with its composition. In crystals that vary in composition from core to rim (not uncommon in plagioclase), it produces a distinctive effect when crystals are viewed between crossed polars.
Examine the granite thin section between crossed polars. In View 3, on rotation, the plagioclase crystal shows varying extinction between a central zone and a marginal zone. This effect is due to progressive compositional variation, often called zoning, which may arise due to mineral growth or to diffusion of elements (as in olivines). In this case, when the rotation is at about 65° it is possible to see 'ghosts' of crystal faces, indicating growth zoning and the development in stages of a well-shaped crystal until it finally filled in the remaining space. Strongly developed concentric zoning can often be a distinctive feature of plagioclase.
In coloured minerals, zoning can show up as concentric colour banding. This is a feature of some pyroxenes.
