2.2.1 Soil pH
pH (a measure of acidity or alkalinity) is an important environmental factor, particularly in soils. Soil is derived partly from accumulated decaying vegetation and partly from broken up fragments of the underlying rocks. Soil pH is determined by both these components and also by the water that fills the spaces between solid soil particles.
How might you expect the pH of soil overlying limestone (or chalk, which is a particular form of limestone) to compare with that of soil overlying sandstone? The soils would have different pH values. Limestone is a calcareous rock rich in carbonates (usually calcite), which gives rise to soils of pH of 7 and above. Sandstones contain much silica and so give rise to neutral or slightly acid soils, where the pH can be as low as 3.5.
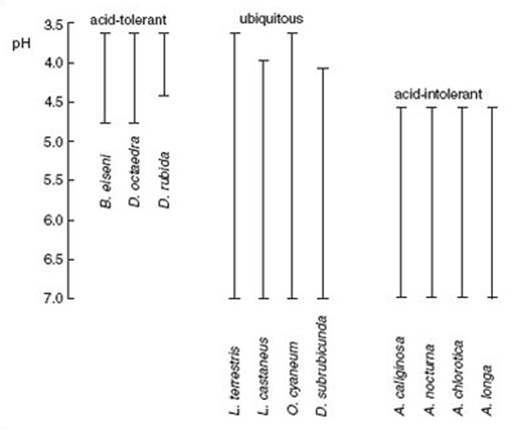
Figure 22 shows the distribution of earthworm species in soils of different pH. You can see that some species have wide pH tolerances, whereas others have much narrower ranges, being specialist species of either acidic or more neutral soils. Species of plants and animals that are found only in alkaline environments are termed calcicoles (i.e. organisms that like a calcium-rich environment), whereas those that dislike these soils are called calcifuges (i.e. calcium escapers).
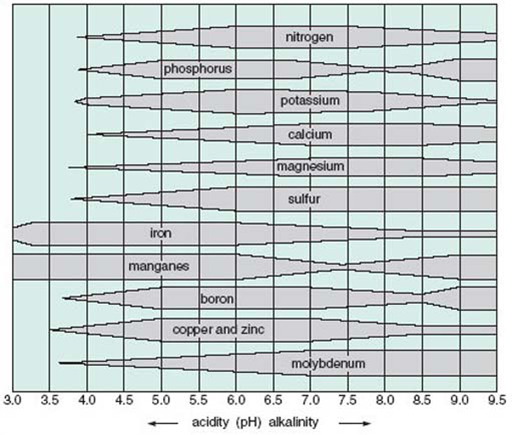
pH is a critical factor for plants, if only because it affects the solubility of mineral nutrients and hence their availability to plants. Figure 23 shows, in the form of ‘kite’ diagrams, the relative availability of several minerals at different pH values. A mineral is most available at pH values where its ‘kite’ is widest and least available where its ‘kite’ is narrowest or non-existent.
Question 6
Which minerals are most available, and which are least available, (a) at pH 7.5–8.5 and (b) at pH 3.5–4.5?
Answer
(a) At pH 7.5–8.5, molybdenum and sulfur are most available, whereas iron and phosphorus are least available. (Fair amounts of magnesium, calcium and nitrogen are also available, but there is comparatively little boron, copper, zinc or manganese.)
(b) In very acid soils of pH 3.5–4.5, manganese and iron are most available, but hardly any of the other minerals listed are available in significant amounts.
Where minerals are absent at low pH, it is often the result of their high solubility in water at these pH values. As a consequence, they move down through the soil dissolved in water (i.e. they are leached) and so are taken into groundwater well below the level of plant roots. In contrast, phosphorus, manganese and boron are locked up in insoluble iron and aluminium phosphates at around pH 7.5. Plants growing on these soils can suffer from deficiency diseases (for example, phosphorus deficiency, which shows as yellowing of the leaves). This is particularly true of plants that are not adapted to grow at high pH. Phosphorus is also unavailable at low pH; again it is locked up as insoluble aluminium phosphate.
Limestone-derived soils may have very high pH values – 8 or above – particularly where the soil is shallow and directly overlies carbonate rock. Very few acid grasslands have a pH as low as 4.5. However, low pH values are a feature of moorlands characterised by heather (Calluna vulgaris) and bracken (Pteridium aquilinum), and also of upland bogs characterised by rushes (Juncus spp.) and cotton sedge (Eriophorum vaginatum), where the pH can be below 5.5.
The reasons why plants show preferences for acid or alkaline soils are not always simple, i.e. attributable solely to nutrient availability. For instance, some minerals can be toxic. Thus calcicoles grown on acid soils suffer from aluminium and other toxicities that cause stunting of their root systems. This stunting reduces nutrient uptake as a secondary effect.
