3.2.1 Polyacids
A large variety of phosphorus acids are derived from 'polyacids', which contain two or more acidic phosphorus centres (see Section 3.3).
Oxoacids by definition contain a covalent AO-H bond, which can dissociate to give a proton and an oxoanion:
There may also be one or more terminal oxygen atoms, so the general formula of oxoacids is A(O)t(OH)n, where t can equal 0. By this formulation, sulfuric acid, H2SO4, is written as S(O)2(OH)2 (where t = 2, n = 2), and boric acid as B(OH)3 (where t = 0, n = 3).
What are the values of t and n for phosphoric acid?
From Table 3, the formula of phosphoric acid can be rewritten as P(O)(OH)3, giving t = 1 and n = 3.
These covalent hydroxo compounds have available a wide range of structural possibilities, which is the reason for the existence of a relatively large number of oxoacids. The variables are as follows:
- There may be several -OH groups in the acid, each one of which can dissociate to form a proton and an oxoanion. For example, in the three successive ionisations of phosphoric acid, H3PO4 (Equations 21-23): each of the species on the left-hand side of the three equations is a different oxoacid, and each of the equilibrium has a different dissociation constant.
- The equilibrium constant for dissociation of the first proton (stage 1), or the 'first dissociation constant', is given the symbol K1 (7.5 × 10−3 mol l−1). The second dissociation constant is K2 (6.2 × 10−8 mol l−1). The third dissociation constant is given the symbol K3 (1.0 × 10−12 mol l−1).
- Oxoacids may undergo condensation reactions to form dimers, trimers and polymers. The term 'condensation' refers to the reaction of two or more molecules to form a larger molecule, with the elimination of a small molecule, most often water. Thus, phosphoric acid, H3PO4, can self-condense to produce diphosphoric acid and triphosphoric acid:
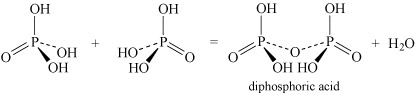 Equation 26
Equation 26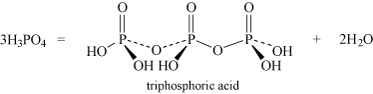 Equation 27
Equation 27It can also form higher polymers described by the general term 'metaphosphoric acid'.
- The central element, A, may exist in more than one oxidation number. Remember, oxygen stabilises high oxidation numbers. In fact, the range of oxidation numbers found in compounds with oxygen is wider than that with any other element except fluorine. Phosphorus, for example, forms oxoacids in oxidation numbers +5, +3 and +1.
- A final complication, arising with sulfur, is that this Group 16 element can take the place of oxygen in an oxoacid, so that there is the possibility of one or more S-S bonds, in what is then called a thioacid.
