4 Cations in water
Sodium, potassium, magnesium and calcium are found in natural water (Table 1). The most important sources of calcium are the mineral deposits of calcium carbonate, CaCO3, which are formed from the fossilised remains of long-dead marine organisms. Examples include the minerals limestone and chalk.
Dissolved carbon dioxide makes rainwater slightly acid.
Suggest an equation for the dissolution of carbon dioxide in water.
- CO2(g) + H2O(l) = H2CO3(aq)(Equation 32)
A carbonic acid solution is weakly acidic:
H2CO3(aq) = H+(aq) + HCO3−(aq)(Equation 33)Consequently, the rock limestone is very slightly soluble in rain:
CaCO3(s) + H+(aq) = Ca2+(aq) + HCO3−(aq)(Equation 34)
This accounts for the levels of both calcium and bicarbonate (HCO3−) ions seen in natural water samples (Table 1). Furthermore, this dissolution of calcium carbonate contributes to the hardness of water.
Water hardness is often due to the presence of dissolved calcium and magnesium salts. For instance, calcium hydrogen carbonate upon heating is converted to calcium carbonate. This calcium carbonate is insoluble and deposits in appliances, such as kettles.
Human derived acid rain can weather minerals by, for instance, converting calcium carbonate (Figure 15) into calcium sulfate, CaSO4, leading to harder water. The resulting volume change leads to surface cracking and new conduits for water to percolate. Freezing of this water leads to further damage. Such acid rain can arise from the combustion of sulfur-containing fuels because this yields sulfur dioxide, SO2, which upon oxidation can yield sulfuric acid. Consequently sulfur compounds are removed from fuels before combustion where possible, often using zeolites.
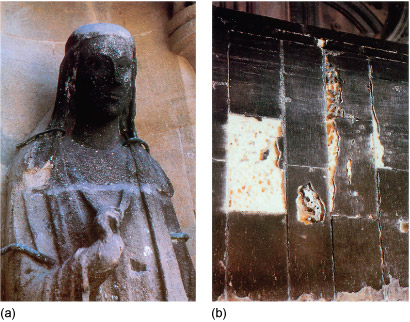
Suggest an equation for the reaction of calcium carbonate with sulfuric acid.
- CaCO3(s) + H2SO4(aq) = Ca2+(aq) + SO42−(aq) + H2O(l) + CO2(g)(Equation 35)
