4.1.2 Aluminium and biology
Aluminium minerals such as bauxite are biologically unavailable due to their insolubility in water. In the course of evolution, this would inevitably have limited the bioavailability of aluminium to living organisms. Aluminium is consequently not an essential element for humans in their normal metabolism. However small amounts of aluminium are found in most people's diet, let us now consider how this might arise?
Watch Video 6. Determine how aluminium reacts with both acid and alkali.
Download this video clip.Video player: Video 6Transcript: Video 6 Reaction of aluminium with acid and alkali.
End transcript: Video 6 Reaction of aluminium with acid and alkali.NARRATORAluminum is an amphoteric metal. It reacts with both acids and alkalis. On the left is an aqueous solution of sodium hydroxide. And on the right is dilute hydrochloric acid. Both reactions produce hydrogen.Video 6 Reaction of aluminium with acid and alkali.Interactive feature not available in single page view (see it in standard view).Aluminum is soluble in both acids and alkalis, dissolving in both hydrochloric acid and sodium hydroxide, liberating hydrogen and finally giving clear, colourless solutions:
2Al(s) + 6H+(aq) = 2Al3+(aq) + 3H2(g)(Equation 45)2Al(s) + 6H2O(l) + 2OH−(aq) = [Al(OH)4]−(aq) + 3H2(g)(Equation 46)
Aluminium metal forms a protective oxide film upon exposure to the air and so does not react further. Therefore, as aluminium is fairly inert, it is commonly used in cookware, especially as it is an excellent conductor of heat.
How might aluminium in cookware be solubilised? Also, consider if aluminium(III) is a hard or a soft cation.
Aluminium can be solubilised from cookware by heating acidic solutions, such as those containing citric acid, Structure 11). Aluminium(III) is a hard cation, similar to the iron(III) ion. Consequently it is bound and solubilised by hard chelating ligands in food such as citric acid.
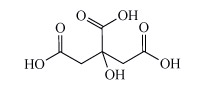 Structure 11
Structure 11
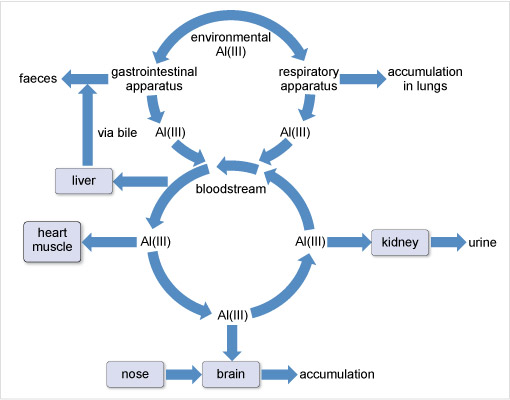
Aluminium has been associated with several neurodegenerative diseases although its role remains controversial. The World Health Organization (2015) sets a tolerable daily intake of aluminium for a 60 kg adult at 60 mg. For most people, the mass actually ingested daily is about 10 mg. This aluminium is mostly excreted in the faeces and is not taken up by the body. That which passes across the gastrointestinal barrier into the blood stream is dealt with by the kidneys (Figure 18). However, there is a small accumulation in the whole body, including the brain and lungs.
There is no doubt that aluminium can damage people with impaired kidney function. The condition called dialysis dementia was first noticed in patients who had received long-term haemodialysis for renal failure. Its symptoms included speech disorders, memory loss, convulsions and seizures, followed, in some cases, by death within a year. The incidence of the disease was highest when the municipal water used in the dialysis contained high concentrations of aluminium. Aluminium is therefore considered a potential neurotoxin.