3.3.2 Adenosine triphosphate
After deoxyribonucleic acid, DNA (which is a phosphodiester), adenosine triphosphate (ATP, Structure 10), is probably the most important phosphorus-containing molecule in the human body.
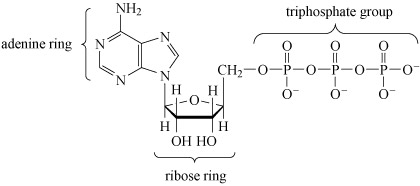
ATP is used to drive many biochemical reactions and cellular processes that require the input of energy; these include cell division and muscle contraction.
Energy is obtained from ATP when it is hydrolysed to adenosine diphosphate (ADP) and free phosphate ions. Equation 31 is thermodynamically favourable, having a free energy change,
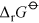 , of about −140 kJ mol−1.
, of about −140 kJ mol−1.
Note that when bonded together the adenine and ribose rings are referred to as adenosine.
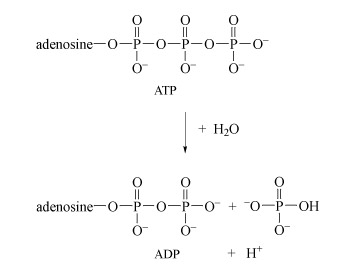
ATP is synthesised by the reverse process - the addition of phosphate to ADP. The energy input for this reaction comes from the breakdown of organic fuel molecules, such as glucose.
The negative charge on ATP, ADP and DNA is counterbalanced by cations, usually Mg2+. Hence, both ATP and DNA may be regarded as magnesium complexes.
