3.3.1 Polyphosphates
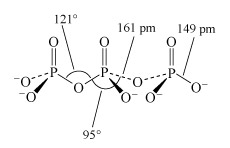
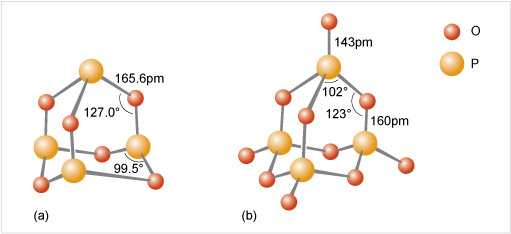
Phosphate tetrahedra, PO43−, can link up by oxygen-sharing to give polyphosphates in the form of rings and chains (Figures 12 and 13). The phosphates, unlike the silicates, contain a central atom with a maximum valency of five. Thus, the maximum number of oxygen atoms that each tetrahedron can share is three, since there must always be one vertex of the tetrahedron that is a terminal oxygen, bound as P=O.
Consider phosphoric oxide, P4O10 (Figure 12b), how many P-O-P linkages does each phosphorus exhibit?
Each phosphorus atom forms the maximum three P-O-P linkages.
Condensed phosphates can be formed if phosphoric oxide is treated with limited amounts of water; or by dehydrating phosphorus oxoacids or their salts by heating.
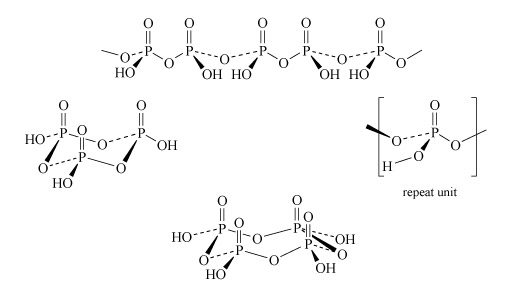
Two molecules of phosphoric acid can condense by loss of a water molecule; the two tetrahedra share one oxygen, giving an acid of formula H4P2O7, known as 'pyrophosphoric', or (more correctly) diphosphoric acid. In practice, this can be obtained by heating phosphoric acid ('pyro' comes from the Greek word for fire). Continuation of this process gives triphosphoric acid, H5P3O10, and, eventually, chain polymers called metaphosphoric acids, which contain repeating [HPO3] units. In Figure 13 the ring polymers which are also shown are also called metaphosphoric acids.
The P-O-P link in polyphosphates is readily hydrolysed; in excess water, metaphosphates revert to orthophosphate. So, unlike the condensed silicates, polyphosphates are never found as minerals. The hydrolysis of polyphosphates is an important source of energy within the body (Section 3.3.2).
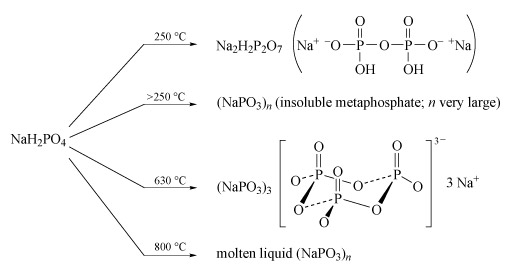
Sodium polyphosphates are the best known; the general reaction for their formation is the dehydration of sodium dihydrogen phosphate by heating. The temperature of dehydration controls the nature of the product (Figure 14).
- Partial dehydration at low temperature (c. 250 °C) produces disodium dihydrogen diphosphate, Na2H2P2O7, a substance used in baking powders as a slow-acting acid for the controlled release of CO2 gas, which produces the aerated texture of cakes.
- Long-chain polyphosphates are produced by heating NaH2PO4 above 250 °C.
- The sodium salt Na3P3O9, containing the cyclic P3O93− anion, is formed when NaH2PO4 is heated to 600-640 °C and the melt then maintained at 500 °C.
- High-temperature dehydration (800 °C) above the melting temperature of NaH2PO4 (628 °C) yields a liquid with very high relative molecular mass, which gives slightly soluble solids of formula (NaPO3)n:
nNaH2PO4 = (NaPO3)n + nH2O(Equation 29)
- Heating together a 2 : 1 mixture of Na2HPO4 and NaH2PO4 at 450 °C produces a chain of only three phosphate units:2Na2HPO4 + NaH2PO4 = Na5P3O10 + 2H2O(Equation 30)
This triphosphate ion, P3O105−, is illustrated in Structure 9.