2.2 Specific heat capacity
When energy reaches the surface of an object, the amount the object heats up is determined by its specific heat capacity. This is a measure of how much energy it takes to raise the temperature of 1 kg of a particular substance by 1 °C. A lower specific heat capacity means that it takes less energy to heat up something, and vice versa. Although the term may be unfamiliar, the concept most likely is not.
Activity 2 The effect of specific heat capacity
On a very hot sunny day on a table outside in the sunshine there is a glass containing 1 kg of water (i.e. 1 litre), a 1 kg piece of cork and a 1 kg piece of iron. Ignore the effects of albedo and assume that all three items absorb the same amount of energy from the Sun. Which will be the hottest after 1 hour, and which the coolest? (Ignore all sources of heat except that received directly from the Sun.)
Discussion
You probably recognised that the 1 kg of iron would be the hottest. It does not take very much heat energy to change the temperature of the iron because it has a low specific heat capacity. The other two items are harder to place, but the cork will be cooler than the iron, and the water, which has the highest specific heat capacity, will be the coolest item on the table.
Water has an extremely high specific heat capacity and it takes a vast amount of energy to heat it. This is why virtually all car engines use water in their cooling systems.
Taking into account the combined effects of albedo and specific heat capacity, even two adjacent areas such as a beach and the sea lapping on it will heat up by different amounts on a sunny day.
Areas with lower heat capacities and lower albedos heat up more. This heat is transferred to the air above, so in these areas it will rise at a faster rate, whilst in cooler areas the air sinks. The rising and sinking air drives horizontal winds much as in Figure 4, although on a planetary scale.
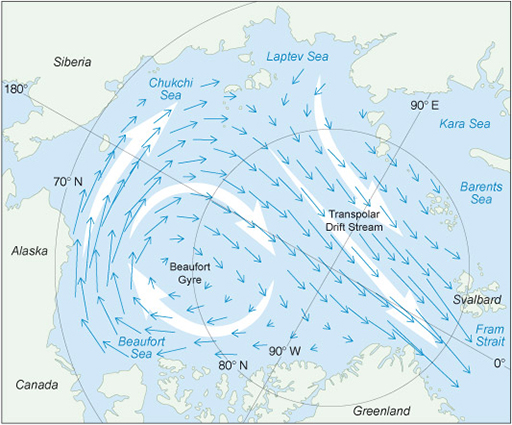
Sea ice cover is also constantly moving. It is pushed by the winds and ocean currents, and drifts in the pattern shown in Figure 5.