2 Anatomical imaging using X-rays
X-rays are routinely used in diagnosis – for example, in examining broken bones. They are part of the electromagnetic spectrum and have the potential to interact with matter, either atoms or molecules. The nature of the interaction depends on the energy of the radiation concerned.
-
In which part of the electromagnetic spectrum are X-rays found?
-
X-rays, with wavelengths in the range 0.01–10 nm, are higher energy than ultraviolet, but lower energy than gamma radiation.
As X-rays pass through matter they may:
- go straight through unimpeded
- be absorbed
- be scattered and carry on in a slightly different direction.
Absorption of X-rays can excite the core electrons in an atom, giving them sufficient energy to be ejected from the atom; as such, X-rays are examples of ionising radiation.
-
What are core electrons in an atom?
-
These are the electrons that are not involved in bonding. For example, in nitrogen, which has the electronic configuration , the electrons are core electrons. The remaining five electrons, which are involved in bonding, are the valence electrons.
The greater the atomic number of an element, the more strongly it absorbs X-rays.
Similarly, the scattering of X-rays also increases with increasing atomic number; which of these processes is the dominant one will depend on the energy range used for imaging.
In addition, the denser the matter, the more opportunities there are for interactions leading to absorption or scattering. In this context, you’ll often come across the term attenuation: the gradual loss of intensity of radiation as it passes through a particular medium.
-
At this point, pause and list the factors that contribute to the overall attenuation as radiation travels through a sample.
-
Atomic number, density and, of course, the thickness of the sample.
To achieve a good image where there is a clear contrast between the components, there must be a difference in the attenuation between the different tissues.
Most body tissue is made from water and carbon-based polymers containing low atomic-number atoms – mainly carbon, nitrogen, oxygen and hydrogen.
-
Bone absorbs X-rays more strongly than the surrounding soft tissue (Figure 1). Why is this?
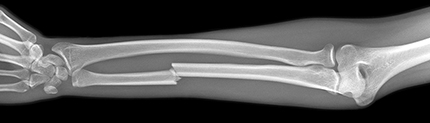 Figure 1 X-ray of a broken bone.
Figure 1 X-ray of a broken bone. -
Bone not only contains elements with higher atomic number than the surrounding soft tissue, such as calcium and phosphorus, but also is denser – a solid with closely bonded atoms.
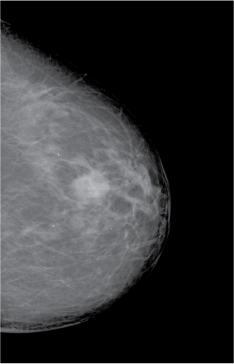
Some types of tissue – for example breast tumours, which are denser than the surrounding tissue – can also be differentiated by X-rays (Figure 2).