The 1,2-intrastrand cross-links
It is thought that the 1,2-intrastrand cross-links are important to anticancer activity because they are the major adducts formed, and because clinically inactive compounds, such as the trans-dichlorodiammineplatinum(II) (transplatin), fail to form these cross-links.
The mechanism of the reaction of cisplatin with DNA is shown in Figure 21.
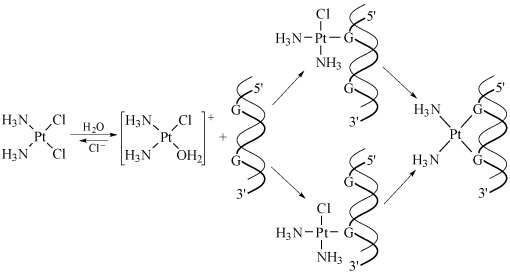
Kinetics is very important in this sequence of steps. The aquation of cisplatin is the slow, rate-limiting step, and the reaction of the cationic platinum complex with the DNA strand is fast.
Hydrogen-bonding from NH3 and OH2 ligands to the phosphate backbone of DNA is possibly important in orientating the platinum complex.
The structural studies of cisplatin binding to oligonucleotides (see the previous section) show that different adducts distort the DNA in different ways, as discussed in the next video.
Transcript: Video 9 The cisplatin story: Part 4. (3:47 min)
NARRATOR: More unusually, a very small amount of the cisplatin – less than five per cent – bonded to the DNA by straddling the two strands, making what is known as an interstrand adduct. The transplatin simply has the wrong geometry to form the linkages. Was this the beginning of the explanation? A more precise technology, such as X-ray crystallography, was needed to sort things out. But the great problem here is in growing good quality, single crystals.
STEPHEN LIPPARD: A major breakthrough in the laboratory, with respect to getting X-ray crystallographic information, came in the mid 1980s when a graduate student in my group – former grad student in my group, Suzanne Sherman – was successful in crystallising the smallest building block on DNA bound to cisplatin.
NARRATOR: The crystalline DNA–platinum adduct was needed for this machine – the X-ray diffractometer. The wavelength of X-rays is comparable in size to the distance between planes of atoms in a crystal, and the regular spacing inside the crystal diffracts the X-rays. Each plane of atoms in the crystal reflects the X-ray, producing a spot on a photographic plate.
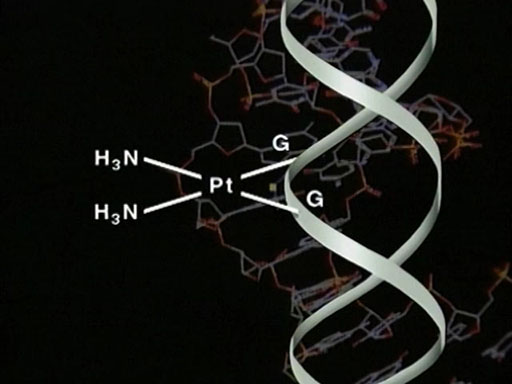
This, at first sight rather unpromising, pattern of spots is the end result. Analysing both the position of the thousands of spots and their intensity produces these dramatic 3D pictures of the molecule itself, where the position of each atom is known precisely. The first structure determined was of cisplatin bound to a single-stranded DNA. And it wasn’t until 1995 that Lippard’s group determined the crystal structure for the double-stranded DNA complex that we see here.
Notice that cisplatin binds in the larger major groove. The platinum is still essentially square-planar, although the guanines are no longer parallel. What was this doing to the structure of the DNA molecule itself?
STEPHEN LIPPARD: The DNA molecule underwent some rather interesting and profound structural changes. For one thing, it underwent a rather significant bend, ranging from 50 to 80 degrees off of the linear direction that it ordinarily takes. For another – and probably this is the most significant – is that the minor groove of the DNA was substantially widened from the normal value in so-called B or classical B form DNA of about 5 to 6 angstroms up to 10 or 11 angstroms.
So, opposite the platinum lesion was a widened, flattened, minor groove, producing a very different type of external structure than the one that one would find in DNA containing no chemical modification. And we believe that it is this altered structure of DNA that leads to the recognition of other factors in the cell, which ultimately produce the anticancer activity.
LLOYD KELLAND: It’s a very complicated area. The cisplatin binds to DNA and forms a variety of adducts on DNA. About 90 per cent of them – much the majority – are the intrastrand adducts on the same strand of DNA, primarily against adjacent guanines on the DNA. But there are also interstrand cross-links that cross the two strands of DNA, of which maybe only two or so per cent, but there are a group of people who believe that they are the more important, in terms of biological significance, whereas there are another group of people who believe that it’s the intrastrand adduct, because of the higher numbers, that think they’re the more important.
-
Which technique was used to determine the nature of the binding in DNA, and what practical issues did it present?
-
X‑ray crystallography – you may recall this is a technique for determining the internal structure of solids. A specific arrangement of atoms will produce a unique diffraction pattern, which acts as a ‘fingerprint’ for a particular compound.
The necessity to produce single crystals of the cisplatin–DNA adduct proved a challenge, solved by the practical ingenuity of one of Professor Lippard’s students.
The main observed effects of 1,2-intrastrand cross-links are:
- a bend towards the major groove of about 35–40°, shown in Structure 12
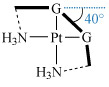 Structure 12
Structure 12 - unwinding of the duplex by about 20°
- widening of the shallow minor groove
- distortion of the Watson–Crick base pairing.
This all leads to destabilisation of the duplex, which in turn blocks replication and inhibits transcription. Replication stops at sites corresponding to one nucleotide preceding the first Pt–G residue and at positions opposite the two Pt–G residues.
You will now look at some experimental evidence for this.
A method for separating macromolecules and their fragments is gel electrophoresis. This is based on the principle that molecules having different sizes or charges will move through a gel under the influence of an electric field to different extents – small molecules move more easily than large ones. In fact, you will see the technique being used in the laboratory in the next section.
Figure 22 shows gel electrophoresis data obtained during a kinetic study of the effect of a cis-GG adduct on DNA polymerisation by HIV-1 reverse transcriptase.
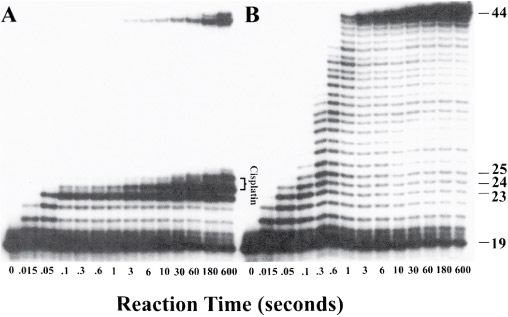
In Figure 22, panel A shows the fragments generated by enzymatic replication of a DNA duplex containing a site-specific cross-link at G(24)/G(25). Polymerisation is blocked by platination of the substrate. Panel B depicts results for an unmodified DNA probe.