5 Cancer therapy: the cisplatin story
The most effective drugs for treating certain forms of cancer are a series of platinum-containing complexes.
For example, they have transformed the statistics of testicular cancer survival from a rare chance up to the 1970s to around a 90% survival rate today. But the discovery of their efficacy is one of serendipity.
The videos that follow throughout this course summarise the history and development of these drugs, with interviews with key players in the field. In the first video, you will meet Barnett Rosenberg (1926–2009), a physicist who had noted similarities between the appearance of magnetic lines of force and a cell when it is dividing. You will see how this led to the experiment that established the anticancer properties of platinum.

Transcript: Video 6 The cisplatin story: Part 1. (6:32 min)
NARRATOR: Fundamental research has led to a most amazing anticancer drug discovery here at Michigan State University. Professor Barnett Rosenberg is a physicist interested in electric and magnetic fields. But his curiosity was aroused by a picture in a biology textbook.
BARNETT ROSENBERG: Physicists rarely have any training or background in biology. But this picture was a very fascinating one because it looked very much like, to a physicist, what you’d see if you put a bar magnet on the table and put a piece of paper on it and covered it with iron filings, and you get that lovely pattern that we call a dipole field.
A dipole magnetic field, or a dipole electric field, they both share the same patterns. Now the curious thing is, is that this was a picture of a cell in the process of dividing. And it showed these lovely structures of the dipole field. And I wondered whether there might not be a connection between the dipole field and the division process.
To test this – and it had been suggested by other people but it had never been tested. So we decided to run a test on it.
NARRATOR: The test equipment he assembled was a combination of electronics and a biological apparatus to grow cells in. Cells of this bacterium E. coli were being cultivated within an electric field. But instead of increased numbers of bacteria, what they found surprised them. The bacteria had instead formed into long strands. What was causing this?
BARNETT ROSENBERG: We concluded finally that it had nothing to do with the bacteria, but it was something that the electric field, which was brought in with platinum electrodes into the chamber where the bacteria were growing, was somehow reacting to produce a chemical that went into the solution. And it was this chemical that was causing these enormously long filaments, which is what we saw under the microscope when we looked at the bacteria coming out of the growth chamber. And it stopped cell division.
Well, stopping cell division is precisely what you want to do if you can control the type of cell if you want to fight cancer. Because cancer is runaway cell division. And so we had initially the idea that this might give us some ability to handle cells, or control the growth of cells, by the electric field effect. But now what we found was, instead of a physical effect, a chemical effect.
NARRATOR: If it was a chemical effect, the culprit had to be within the growth chamber. It was narrowed down to a molecule made by the chemical reaction between the nutrient ammonium chloride and the electrodes, which were made of platinum.
BARNETT ROSENBERG: Platinum could be one of the possibilities, although we all believed that platinum was inert in the biologic environment. Then, when we had the chemicals isolated, we then tried to find out which particular one of the platinum/ammonium chloride chemicals it was. And we tried finally to isolate it. It turned out to be a neutral molecule. And it turned out to have a very specific structure.
NARRATOR: This is the formula of the chemical they had produced. The platinum had reacted with the ammonium chloride, and bonded to two chlorides and two ammonia groups. This molecule could have one of two flat structures where the platinum is surrounded by four ligands.
This shape is known as square planar. One form has cis chloride ligands, which means that they are on the same side of the molecule, at right angles to each other. This is commonly known as cisplatin. The other form has the same chemical formula but now the chloride ligands are trans or opposite one another. It’s known as transplatin. Only the cis form of this chemical was active against cell growth.
BARNETT ROSENBERG: We knew we had a method, then, of controlling cell division. It was not a physical method. We were disappointed in that. But we had a chemical method. And, therefore, we went back to our original concept. Could we then control the growth of cancer cells by using this?
NARRATOR: Next experiment was to inject doses of cisplatin into tumours in mice and compare results against a control group. The tumour decreased dramatically in size. The drug worked.
BARNETT ROSENBERG: So this became, then, the focus of our interest because now we could treat a cancer in a mouse. The question was, would it work in humans?
NARRATOR: Professor Rosenberg and his colleagues wrote up the reports in the science journals. And the medical research community went into action on both sides of the Atlantic.
LLOYD KELLAND: The drug then very rapidly, particularly for these days, went into clinical trial in patients, both in the United States and in Europe. And the first hospital to look at cisplatin in Europe was in fact the Marsden Hospital, next door to here, where Dr Eve Wiltshaw very rapidly showed that the drug had activity in ovarian cancer in particular. And, alongside that, other trials showed that the drug was active in men with testicular cancer.
Before the advent of cisplatin, especially in the case of men with testicular cancer, the cure rate was something like one in ten. When cisplatin-containing regimes came into clinical practice, the cure rate went up to something like nine in ten. So it had a dramatic effect and it was a very exciting time. It was probably the most active new cancer drug found in that period.
As you saw in Video 6, Rosenberg wondered if an electric field would affect cell division.
He conducted an experiment on the bacterium Escherichia coli, subjecting it to a field in an electric cell containing platinum electrodes, with a growth medium of ammonium chloride.
-
What did Rosenberg and his group observe?
-
They found that cell growth was not affected, but that cell division was curtailed, with the result that he observed the growth of long filaments (Figure 16). He realised that the inhibition of cell division could be a very important discovery for cancer.
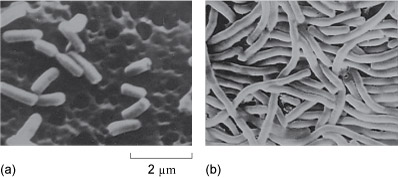 Figure 16 Scanning electron micrographs of E. coli grown in medium containing a few parts per million of cis-diamminedichloroplatinum(II). (The same magnification is used in each image.) The platinum drug has inhibited cell division (a), but not growth (b), leading to long filaments.
Figure 16 Scanning electron micrographs of E. coli grown in medium containing a few parts per million of cis-diamminedichloroplatinum(II). (The same magnification is used in each image.) The platinum drug has inhibited cell division (a), but not growth (b), leading to long filaments.
The initial assumption was that the platinum electrodes were inert but further studies showed they react with NH4Cl to give cisplatin, [Pt(NH3)2Cl2].
-
Active content not displayed. This content requires JavaScript to be enabled.Interactive feature not available in single page view (see it in standard view).
-
Sketch the structure of cisplatin. What is its geometry?
-
Cisplatin (Structure 3) is a square-planar complex.
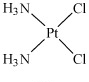 Structure 3
Structure 3
Early laboratory experiments showed cisplatin to be active against tumours in mice, and in 1971 it entered clinical trials. It was finally approved for clinical use in the USA in 1978. Now it and its derivative drugs are used very successfully not only against testicular and ovarian cancer, but also for head and neck, bladder, lung and cervical cancers, and lymphoma, melanoma and osteosarcoma.
You will now consider the mechanism of the anticancer activity of cisplatin.
