5.6 Photovoltaics in the context of renewable energy
Energy use is fundamental to our modern economy and society. Energy enables manufacturing, communications, transportation, entertainment, and the domestic and work environments. However, its generation from nuclear and fossil fuels is presenting increasing problems (see Energy from atoms and molecules ). Long-term side effects and limitations in the amount of fossil fuel available mean that we must develop new attitudes to energy generation, and also new technologies for energy generation. Our future management of energy needs to be radically different from what took place in the twentieth century. What is needed is an energy supply that is 'renewable'. In fact, the best we can hope for is something that appears on our time scales to be everlasting – rather like coal and oil must have looked a few generations ago. So, what we mean by 'renewable' is a resource that is naturally replenished over a short time span.
Energy from atoms and molecules
There are a number of ways to get energy from atoms and molecules. Here are three. Burn a hydrocarbon or other organic substance; break up the nuclei of massive atoms; fuse together nuclei of some of the smaller atoms. The first is common place, but not without an environmental impact. The second is the nuclear industry's serious business. The third is incredibly difficult to control and is the subject of lengthy and expensive research programmes.
- a.Burning releases energy, i.e. things get hot as they burn. This is essentially a rapid process involving chemical combination with oxygen; if the reaction is too rapid then an explosion is likely to occur.
A simple reaction to describe is the burning of carbon, the major component of coal, forming carbon dioxide.
C + O 2
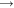 CO 2
CO 2Two other simple reactions are the burning of hydrogen (making water) and the burning of methane.
2H 2 + O 2
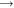 2H 2 O
2H 2 OCH 4 + 2O 2
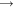 CO 2 + 2H 2 O
CO 2 + 2H 2 OAll we need here is to observe that rearranging the atoms in these reactions releases energy as heat. More complicated hydrocarbons such as fuel oil and aviation spirit burn in much the same way, giving the same energetically preferred products (CO 2 and H 2 O) together with lots of heat.
- b.Very big atoms with nuclei that combine many tens of protons and neutrons are rarely stable, energetically speaking. You know what happens to unstable systems – like a pencil balanced on its end, they are likely to fall into a more stable configuration that has lower energy. Some very big atoms emit radioactive particles as their nucleus shifts around to achieve some lower state of energy. Others, like a type of uranium known as U-235 (a huge atom with a total of 235 neutrons and protons in its nucleus), can be triggered into flying apart by hitting them with neutrons. Huge amounts of energy are released when such massive atoms are split in two. Making use of this requires arranging the nuclear fuel and controlling the neutrons so as to ensure that just enough fission is happening to maintain this reaction at a constant rate. Nuclear power stations achieve this and harness a substantial fraction of the energy released. A commonly quoted figure is that one tonne of uranium fuel can produce the same amount of electricity as 150 000 tonnes of coal.
- c.At the other end of the nuclear scale are the tiny nuclei of hydrogen and helium atoms. Very small nuclei are also not favourable in energy terms, so considerable savings in energy can be made if small nuclei are squeezed together to make bigger nuclei, though enormous forces must be overcome before it is achieved. The process is called nuclear fusion. Making a power station that recovers useful energy from nuclear fusion requires holding small quantities of mind-bogglingly hot gas away from the sides of its container, and finding materials for the container that can remain intact for years despite suffering a very heavy bombardment of neutrons. Although researchers have been able to sustain a fusion reaction for several seconds and extract more energy than was put in, many obstacles still stand in the way of building a power station that can maintain a useful power output over months and years. Fusion processes in the Sun are responsible for its entire output of energy, demonstrating both the incredible potential of nuclear fusion and the extreme conditions associated with the ignition of fusion reactions.
