5 Engineering: pushing back the boundaries
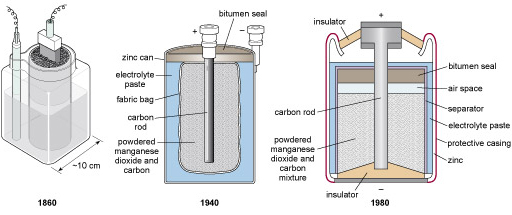
Figure 76 shows a selection of zinc–carbon batteries from different times. There has been a clear trend towards ever more versatile sources of electricity, packing in more energy per kilogram together with improvements in ruggedness and flexibility; at the same time, however, environmental issues have constrained the range of chemicals involved. Over the years the pace of battery development has been set by the requirements of different users. Let's look back briefly to the beginning of industrial-scale electricity to see where the idea of the battery came from.
In the 'Electro-optic Age' at the start of the twenty-first century, digital cameras, mobile communication sets, tablet computers and numerous other gadgets rely on batteries as a sort of 'life support' system. Weight and size are of utmost importance in these devices – they require lightweight (portable) batteries with enough electrical energy to keep them working for at least several hours at a time. Implanted medical devices such as cardiac pacemakers make even greater demands, needing several years of capacity in a battery that cannot be much bigger than a large coin.
In the 1950s, when semiconductor technology first offered radios that were small enough to fit into a pocket, batteries were already sufficiently small that they could be classed as portable. Such portable batteries were thanks to the requirement for a portable energy source for the electric torch or pocket lamp, the invention of which was enabled by the advent of tungsten-filament bulbs – when these appeared in the early 1900s, batteries (though non-portable) were already available. An electric torch needs a steady supply of current, preferably throughout a long lifetime. Dim lights are useless, so lifetime was a major issue for this generation. The shelf life and the after life are critical too. The electricity in a battery comes from harnessing the energy generated by a process of controlled corrosion. It is important for an unused battery to remain in peak condition until it is needed, so the corrosion that will ultimately make it work must be prevented from getting underway before then. In batteries from 50 years ago, the corrosion tended to continue even when the battery remained unused, ultimately resulting in it bursting through its package – good for the torch manufacturer in the 1950s as the corrosion quickly spread, rendering the whole device unserviceable!
Earlier still, the electric telegraph was the first major consumer of electrical energy derived from batteries. The development of the electric telegraph was spurred by the expansion of railways and the requirement for universally agreed time. By the end of the 1800s, telegraphy was calling for improvements in battery systems to give longer-range, higher-reliability signalling through cables that criss-crossed the globe. One might ask, which came first: the battery or the telegraph? The fact that the battery did by several years leaves one wondering just why anyone bothered to devise such a convenient source of electricity without it having any application. There clearly was no real necessity at this stage. Instead, curiosity provided the driving force.