1.2.4 Ammonia synthesis by bulk production
To make high explosives, such as TNT, requires chemicals called 'nitrates'. At the time of the First World War these were obtained from a natural mineral, which was mostly supplied from South America. Germany was cut off from these mineral supplies, so its chemical industry was charged with the task of manufacturing nitrates artificially.
Nitrates are essentially composed of two gaseous elements: nitrogen and oxygen, which are chemically combined. Nitrogen is abundant in the atmosphere, around 76% by mass, but in an unreactive form. The task therefore came down to coaxing atmospheric nitrogen into a more reactive condition. A particularly good method of achieving this is to combine nitrogen with another gaseous element, hydrogen, to form ammonia. The Haber–Bosch process for making ammonia was the outcome of the German war-driven research and enabled Germany to maintain its supplies of munitions. The process is now the mainstay of ammonia production world-wide each year, with ammonia mainly used as the basis of nitrogenous fertiliser. That said, it is also used as a constituent of many household cleaners, as a refrigerant in industrial refrigeration systems, to scrub sulphur dioxide from the burning fossil fuels used in power plants, and for the pulping of wood in the paper industry (Figure 12).

Activity 5 (video)
Watch Video 1 which describes the background to the discovery of how to manufacture ammonia artificially.
Transcript: Video 1 Manufacturing ammonia artificially (2 minutes)
The production of a chemical like ammonia is rather different from the mass production used for a ballpoint pen. This is because the product, ammonia, is not particularly useful in itself: it becomes the starting material in yet further processes. The product does not come out as discrete items, and production may be continuous. This type of production is often referred to as 'bulk production'.
Ammonia production is a good example of engineering as the 'appliance of science', the science in this case being chemistry (see Engineering with atoms ).
Engineering with atoms
When chemistry is applied as a means to an end, where that end is a product with a function, it can be thought of as engineering with atoms. Clearly chemistry is large-scale engineering, and not one atom at a time. The chemical reactions involved often progress without any intervention from humans, indeed some reactions can only be prevented by ensuring that the chemicals involved are kept well apart. However, there is still engineering skill required for chemical manufacture, such as creating the ideal conditions for bringing atoms together to produce the required product, be it a drug, a fertiliser or furniture polish.
True 'atomic engineering' is beginning to emerge in the field of nanotechnology, where atoms and molecules are manipulated singly, in small groups, or in layers a few atoms thick. Many products are coated with thin films for special purposes. One can think of examples such as the anti-reflective coatings on lenses, hard coatings of titanium nitride on drill bits, and self-cleaning glass windows. These are coated with a very thin layer of titanium dioxide that breaks down dirt particles with the help of sunlight and the oxygen in the air, and have been available to consumers since the early 2000s.
Manipulating atoms one by one takes this a step further, and the ability to do this is especially interesting to computer chip manufacturers. In February 2012, a group of researchers at the University of New South Wales announced that they had made a transistor consisting of a single atom of phosphorus. (A transistor is a device used for amplifying and switching electronic signals.) Figure 13 shows the transistor sitting in the space created by removing an atom of silicon from the surface of a silicon crystal. The two wide bands running diagonally across the image are the electrodes; the gap between them, where the phosphorus atom sits, is 20 nanometres wide. This particular transistor would be difficult to use as it behaves well only if cooled to −233 °C, about 40 K above absolute zero, but the ability to create this device will undoubtedly lead to more practical applications.
For the production of ammonia, the elements nitrogen and hydrogen have to be coaxed to react together: ammonia is the product of this reaction under the right conditions. The right proportions are also needed: 1000 kg of ammonia requires 820 kg of nitrogen to react with 180 kg of hydrogen (see Units of mass ). The reactants (i.e. the chemicals that are reacting) are gases, and so is the product. So one constraint on the production system is that it has to supply the ingredients and remove the product in the gaseous state.
Units of mass
Before defining the units of mass it's very important that the distinction between 'mass' and 'weight' is clear. In everyday language we use the term 'weight' when we're really talking about mass.
For example, many people are interested in their body mass index as a measure of health. When I visit my doctor she asks me to stand on the scales (Figure 14) so that she can 'weigh' me. The reading from the scales is 72 kilograms (kg). To calculate my body mass index my doctor also needs to know my height in metres, which is 1.74 m.
Now, when my doctor wants to find my body mass index (BMI), she does the following calculation
Fortunately, this is in the healthy range of 20–25.
You may be wondering why I have put 'weight' in inverted commas and why BMI isn't referred to as 'body weight index'. Well, what my doctor is interested in when I stand on the scales is how much of me there is – that is, my mass. And in SI units we measure mass in kilograms. So, the equation should say
But what the scales are actually doing is measuring the force my body exerts on the scales when I stand on them. This force is produced by the gravitational effect of the Earth pulling on my body and this, in engineering terms, is my weight. Weight is measured in 'newtons' however, the scales are calibrated to give a read out in kilograms.
If my doctor and I were on the Moon, where the effect of gravity is much less than it is on Earth, the force that my body exerts on the scales would be less and my weight would be lower. But my mass would not change – there would still be the same amount of me!
The basic unit of mass is the gram, but it is actually the kilogram (1000 grams) that is standardised and which provides the SI unit for mass. A litre of pure water was defined to have a mass of 1 kilogram. Again the definition was translated into a single prototype, this time as a cylinder of a platinum–iridium alloy, which is dense and uncorrodable. The standard kilogram is actually quite a small cylinder (Figure 15), about 4 cm high and wide, housed in an environmentally controlled safe at the BIPM (the International Bureau of Weights and Measures) in Paris.
Once again – as with length – multiples and subdivisions of the basic unit, the gram, are useful; and, because the SI generally uses a factor of 1000 to define multiples and subdivisions, the prefixes that you met in connection with length are also used with mass.
| Factor | Symbol | |
|---|---|---|
| microgram | × 1/1 000 000 | μg |
| milligram | × 1/1000 | mg |
| gram | 1 | g |
| kilogram | × 1000 | kg |
| tonne | × 1 000 000 | t |
Notice that 1000 kg (a megagram) is actually called a tonne. (Its spelling distinguishes it from the imperial 'ton', though they are very close – to within about 2%. The imperial system of units is discussed further later in this section.)
You will find examples of SI units of mass in everyday situations, often in combination with other units. Listen out for figures describing air pollution in micrograms per cubic metre (μg m −3 ). The legal limit (in 2012 in the UK) for the concentration of alcohol in a driver's blood is 80 mg per 100 cm 3. Chocolate is often sold in bars of 100 g and sugar in 1 kg bags.
Theoretical chemistry provides us with a clear understanding of what conditions are needed to produce ammonia. There is no need to go into great detail here regarding the chemical principles involved, but it is worth looking at their consequences as an example of the fundamental physical laws that constrain much of engineering. Making ammonia is not as simple as stirring the raw materials together in a bucket; and understanding the process by which ammonia is formed allows us to make it as efficiently as possible.
First, the reaction between nitrogen and hydrogen proceeds better at high pressures. If the engineer can provide a high-pressure system for making the ammonia, it will be much more efficient, and so more productive, than one operating at normal atmospheric pressure.
Secondly, chemical reactions often produce heat, and the formation of ammonia certainly does this. However, if the temperature becomes too high, the reaction can become slow; so the engineer can help production by getting rid of the heat generated by the reaction, thereby keeping production high.
Thirdly, it's important to extract the ammonia as it is being made. As more and more ammonia is produced in the reaction chamber, using up the nitrogen and hydrogen, the reaction will slow down and eventually stop. This would happen before all the nitrogen and hydrogen were used up: the presence of the ammonia slows the reaction. Extracting the ammonia product and pumping in more ingredients will keep production continuous.
This is an example where the engineering is driven by the chemistry. Without an understanding of how ammonia is made by this reaction, and how chemical reactions behave in practice, it would be an extremely inefficient process, and indeed perhaps impossible to manufacture ammonia on any viable industrial scale. Yet the chemical knowledge is insufficient without the engineering expertise to build a plant to the required specification: a plant that will fulfil the conditions that I have outlined above.
Having collected the arguments from chemistry on the conditions to make ammonia it remains to ask how to make it quickly. Typically plant will only be economic at production rates of a few thousand tonnes per day. The optimum pressures and temperatures need to be found. That is a job for theory and for development research.
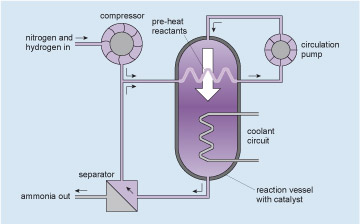
So, with all this science understood, our chemical engineers have to design and build a system that takes advantage of it. Figure 16 indicates the principles of the plant.
The gases at high pressure are pumped around the circuit shown (follow the arrows starting at the circulation pump, which pumps the mixed, but unreacted, ingredients into the reaction vessel). The gases enter the reaction vessel at the top and some of the nitrogen and hydrogen react to form ammonia as they pass over a catalyst within the reaction vessel (see Catalysts and converters ). The mixture leaving the reaction vessel at the bottom passes to the separator where the ammonia product is removed. The unreacted nitrogen and hydrogen, together with enough extra nitrogen and hydrogen injected from the compressor to make up for what has reacted, return to the pump via a heat exchanger within the reaction vessel which both pre-heats the reactants and removes some of the reaction heat. More excess heat is removed from the reaction vessel by the coolant circuit. Thus a continuous production of ammonia is achieved. I will not go into the subtleties of the internal design of the reaction vessel which make the process efficient, nor of the control systems which link the heat and gas flows. Rather I will concentrate on the engineering of the reaction vessel itself.
Catalysts and converters
You may have come across the term catalytic converter , probably in the context of cars and exhaust emissions. A catalyst is something that speeds up or otherwise aids a chemical reaction. A true catalyst doesn't participate in the reaction at all: it just helps it on the way, and is unaffected itself, being left behind as good as new once the reaction is over. In petrol- and diesel-engined cars, a chamber with a platinum-based catalyst is fitted as part of the exhaust system. Some gases in the exhaust, particularly nitrogen oxides and unburnt fuel molecules, decompose into more benign gases when they come into contact with the catalyst.
In the case of ammonia production, iron granules with a large surface area are the usual catalyst.
The ammonia reaction vessel is large, perhaps a cylinder 20 m high by 2 m in diameter, which has a volume of about 63 m 3 (see Units of area and volume ). For efficient performance, the pressure needs to be about 350 times atmospheric pressure (or '350 atmospheres').
The contents of the reaction vessel, at a temperature of several hundred degrees Celsius, include a poisonous gas, ammonia, and an explosive one, hydrogen. The consequences of a leak could be extremely dangerous. So, beyond the primary engineering to provide function, an additional issue here is safety. How do you make a vessel of that size, capable of holding the pressure, with at least six pipes going into it, and guarantee that it is safe? The answer lies largely in 'past practice'. Because pressure vessels have been made for a long time, for many purposes (for example, high-pressure steam boilers in power stations), the 'know how' has gradually developed. Each increment of extra performance demanded from time to time has pushed knowledge a bit further, and the occasional accident serves as a salutary reminder that we don't always get it right.
Units of area and volume
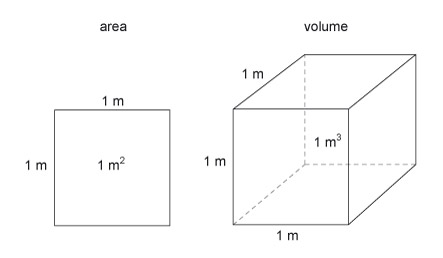
The basic units of area and volume are the 'square metre' (m 2 ) and the 'cubic metre' (m 3 ) (Figure 17). These are simply shapes which have sides that are 1 m in length.
Square metres are inconveniently small for measuring land holdings so the French Revolutionary Committee decided to define 100 m 2 (i.e. 10 m × 10 m) as 1 are (pronounced 'air'). A more frequently used measure of land area is the 'hectare'.
Activity 6 (example)
Use Table 1 in Section 1.2.1 to remind yourself what 'hect' means and then work out how many square metres there are in a hectare.
Answer
'Hect' means × 100, so 1 hectare is 100 are, which is
- 100 × 100 m 2 = 10 000 m 2.
Cubic metres, on the other hand, are inconveniently large for measuring a person's daily intake of food or drink. You will often come across the cubic centimetre, cm 3 (i.e. 1 cm × 1 cm × 1 cm, or 0.01 m × 0.01 m × 0.01 m) or other units derived from the metre. A related unit of volume, the litre, has also been defined and is commonly used to measure volumes of liquid. A litre is the volume of a cube of 1 decimetre – or 10 cm edge length. The symbol for 'litre' is its initial letter, l, but this is so easily confused with the number 1 (for example, eleven litres can be written as 11 l) that it is usually safest to write out the word litre.
An even smaller unit of volume, the millilitre, is also useful. It is one thousandth of a litre. The symbol for millilitre is ml, which is not as ambiguous as the litre symbol.
Activity 7 (self-assessment)
- a.Use Table 2 in Section 1.2.1 to remind yourself what 'deci' means, and then work out how many litres there are in a cubic metre.
- b.A bottle of water has a volume of 500 ml. How many cl is this?
- c.What is the relationship between 1 ml and 1 cm 3 ?
- d.The standard size of a British brick is 215 mm × 102.5 mm × 65 mm. First guess its volume in litres, then calculate it.
- e.How many bricks make a cubic metre of wall (ignoring the mortar)?
Answer
- a.The 'deci' prefix means × 1/10, which is equivalent to ÷10, so there are 10 dm in one metre. Hence,
- 1 m 3 = 10 × 10 × 10 dm 3 = 1000 litres.
- b.The 'milli' prefix means ÷1000. The 'centi' prefix means ÷100. So there are 10 ml in 1 cl and, therefore, 500 ml = 50 cl.
- c.10 cm = 1 dm so
- 10 × 10 × 10 cm 3 = 1 dm 3 = 1 litre.
- So 1000 cm 3 make 1 litre, and hence 1 ml is the same as 1 cm 3.
- d.The dimensions of the brick in cm are 21.5 cm × 10.25 cm × 6.5 cm.
- The volume of the brick in cm 3 is therefore
- 21.5 × 10.25 × 6.5 cm 3
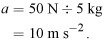 1432 cm 3.
1432 cm 3. - But 1 ml is the same as 1 cm 3 so the volume of the brick is 1432 ml, or 1.432 litres.
- My guess, thinking of a litre box of drink by comparison, was that the brick was 'similar' and would have a volume of about one litre. I was wrong by a factor of 1.432.
- e.There are 1000 litres in a cubic metre. We know from (d) that each brick is 1.432 litres in volume, so we'll need 1000/1.432 = 698.3 bricks; in other words, approximately 700 bricks.