1.7 Localization of signalling proteins
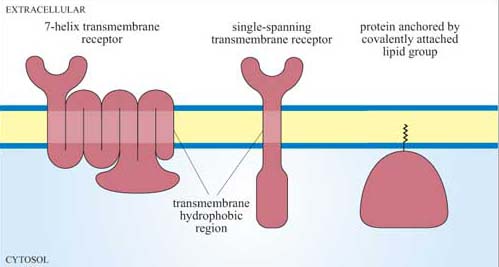
Since signalling proteins cannot diffuse as rapidly as small second messengers, they need be close to their downstream target in order to be able to function. Where they are located with respect to both their subcellular position and their immediate neighbours is therefore vitally important. The plasma membrane is usually the initial location, and proteins can be attached to the plasma membrane in various ways (Figure 10). Many have hydrophobic regions that are inserted into the membrane as the polypeptide is being synthesized (for example, transmembrane receptors).
What post -translational modifications could serve to anchor a signalling protein to the cytosolic side of the plasma membrane?
Covalent addition of a lipid group, prenylation or fatty acylation, tethers proteins to the internal surface of the plasma membrane. The Ras family of G proteins is an example of this type of protein.
The area of the cell membrane near a receptor can become crowded with signalling molecules. Very often, several signalling pathways will need to be activated following binding of the ligand to the membrane receptor, since the cellular response may require multiple changes in cell behaviour (such as a change in cell shape, altered metabolism or changes in gene expression). Many signalling molecules, leading to different signal transduction pathways, will be packed together around the cytoplasmic domain of the receptor, and it is unclear how unwanted signalling outcomes are avoided and how signal specificity is maintained.
One mechanism involves the signalling molecules being arranged on a protein scaffold, such that the proteins are ordered in the correct signalling sequences or, in other words, as a preassembled signalling complex (Figure 11a). This scheme requires one of the signalling components to be able to detach itself from the complex and distribute the signal to other parts of the cell. A similar strategy congregates receptors together with many signalling proteins into specific areas in the plasma membrane such as cholesterol- and glycosphingolipid-rich lipid rafts, which may then be considered as plasma membrane signal initiator units.
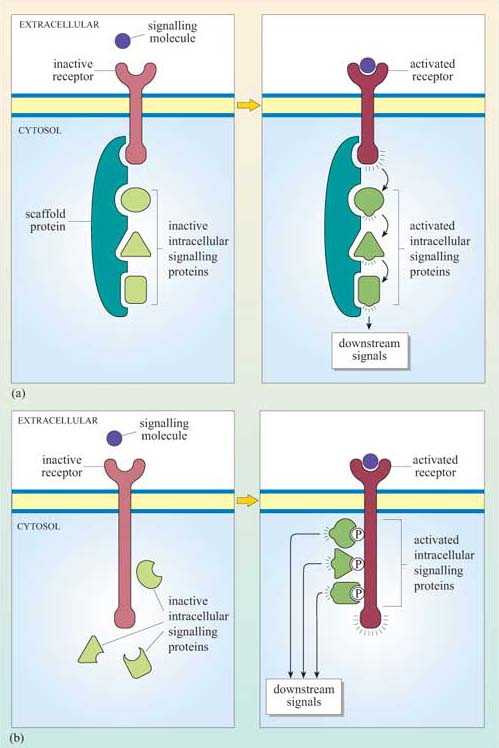
Alternatively, complexes can form transiently, following receptor activation. In this case, the intracellular signalling proteins only assemble once the receptor has bound its extracellular signal molecule (ligand). A common mechanism involves the autophosphorylation of key amino acid residues in the cytoplasmic domain of the receptor after ligand binding. The signalling proteins then recognize and dock onto particular phosphorylated amino acids (Figure 11b). We shall see specific examples of this in Section 3.
At some point, the signal has to be transmitted over a significant distance and between cellular compartments within the cell in order to reach its targets. How does the signal ultimately escape from the cytosolic side of the plasma membrane? As pointed out earlier, one mechanism involves the deployment of small, diffusible second messengers, which also results in amplification of the signal throughout the cell. However, this system lacks specificity in the subcellular target. Alternatively, signalling proteins themselves can be directed specifically to another part of the cell. In order to achieve this, their ‘on switch’ (when they are activated by an upstream signalling molecule) must somehow enable them to be transported. For example, when the signalling enzyme MAP kinase is phosphorylated on tyrosine and threonine residues by its upstream kinase, it translocates from the cytoplasm into the nucleus, where it phosphorylates specific transcription factors and so alters the pattern of gene expression. (The MAP kinase pathway is considered in more detail in Section 3.6.)