3.3.2 Phospholipase C (PLC)
Members of this family of enzymes contain two catalytic domains and several protein binding domains (Figure 13). The PH domain can temporarily tether phospholipase C to the membrane by attachment mainly to PI(3,4)P2.
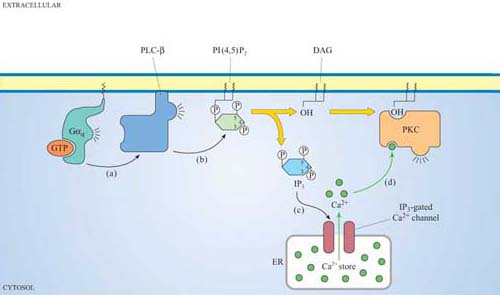
We shall discuss two main isoforms of PLC: PLC-β, which is activated by a subset of trimeric G proteins (Gαq and Gα0), and PLC-γ, which, in contrast, associates with phosphotyrosines on activated RTKs (such as the PDGF and insulin receptors) by means of its SH2 domains. The substrate of both PLC-γ and PLC-β is PI(4,5)P2, which is cleaved by PLC to produce two second messengers: 1,2-diacylglycerol (DAG) consists of linked fatty acyl chains, and so remains in the plasma membrane; inositol 1,4,5-triphosphate (IP3) consists of the phosphorylated inositol ring, which because it is water-soluble is able to diffuse through the cytosol.
Why is IP3 released from the plasma membrane? You may want to look back at the structure of IP3 (shown in Figure 30).
IP3 is hydrophilic and lacks the hydrophobic fatty acyl chains that anchor inositol phospholipids in the plasma membrane.
IP3 binds to IP3-gated calcium channels on the ER membrane, causing Ca2+ stored in the ER to flood into the cytosol. This activates many proteins, but most notably (in this scenario) the protein kinase C family (PKC, so called because of their Ca2+ dependence). Binding of calcium causes PKC to translocate to the membrane (Section 3.4). Full activation of PKC is complicated and depends on the isoform involved, but generally DAG binds to, and helps to activate, protein kinase C. Thus, the two products of PLC activity are acting in a coordinated fashion on the same protein (Figure 32).