3.2 Trimeric G proteins
G proteins are attached to the cytosolic face of the plasma membrane, where they serve as relay proteins between the receptors and their target signalling proteins.
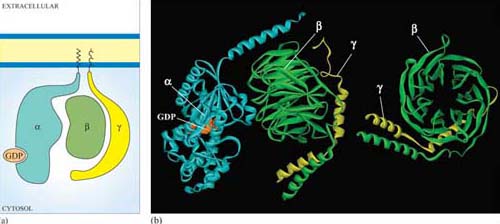
Trimeric G proteins interact with 7TM receptors and are all heterotrimeric, having structurally different α, β and γ subunits. Monomeric G proteins are the small G proteins, such as Ras, which are structurally related to the α subunit of trimeric G proteins.
The three-dimensional structure of trimeric G proteins in their inactive form is shown in Figure 28.
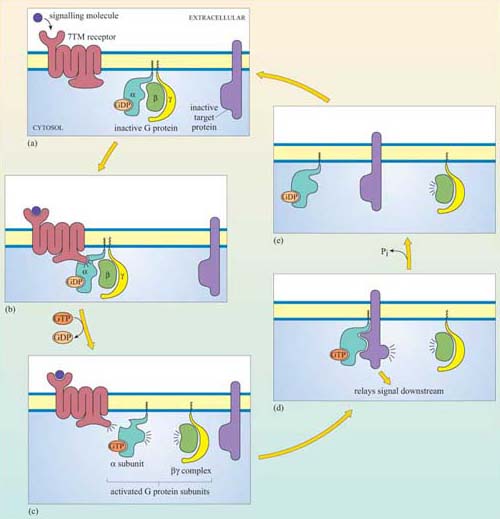
Ligand binding induces a conformational change in the 7TM receptor, which results in the release of GDP and binding of GTP to the α subunit (Figure 29). As a result, the α subunit also changes conformation and becomes activated. This conformational change results in the dissociation of the α subunit from the βγ complex, which also becomes activated, although it does not change conformation itself. The α subunit primarily, and also the βγ complex to a lesser extent, regulate the activity of downstream effector proteins located on the plasma membrane. There are many different α subunits, which can be classified according to sequence similarity, and to which upstream and downstream proteins they interact with (see Table 2 for the most important ones). In fact, the G protein complex is often categorized by the type of α subunit it is formed from; hence you will come across Gαs, Gαi, Gαq, etc. For example, Gαs stimulates adenylyl cyclase, whereas Gαi inhibits it, and Gαq activates PLC-β (see Table 2). There are also different βγ subunits, some of which have been shown to have their own effector function. More generally, though, βγ subunits are thought to stabilize the inactive state of the α subunit.
| Target effector protein | G protein subunit type | Interfering toxin† |
|---|---|---|
| ion channels | regulated by Gαs, Gαi, Gα0 and βγ (for example, Gαi and Gα0 coupled to muscarinic ACh receptor activates K+channels) | |
| adenylyl cyclase | activated by Gαs | cholera toxin |
| inhibited by Gαi | pertussis toxin | |
| cGMP phosphodiesterase | activated by Gαt (transducin) in Photoreceptors | |
| phospholipase C-β | activated by Gαq and Gα0 | |
| phospholipaseA2 | activated by a βγ ? complex | |
| PI 3-kinase | activated by a βγ ? complex | |
| small GTPases | Gα12/13 |
Footnotes
†Cholera toxin and pertussis toxin (from the Bordetella pertussis bacterium, which causes whooping cough) both interfere with the action of G protein α subunits. Cholera toxin locks Gα subunits into an active form and pertussis toxin interferes with Gαi subunits by inhibiting them, making these toxins useful laboratory tools for determining which signalling pathways are activated by GPCRs.G proteins usually remain active for only a short time, which depends mainly on the rate of hydrolysis of GTP to GDP (Figure 29). The intrinsic GTPase activity of the a subunit is quite inefficient by itself. For many cell signalling processes where a rapid turnover rate is necessary (for example, transduction of a photoreceptor activated by visual stimuli), the intrinsic GTPase activity of the α subunit is usually aided by binding of a second protein that enhances the rate of G protein inactivation. This may be either its target protein, ensuring that the α subunit remains active for just as long as it takes to make contact with the target, or a GTPase activating protein (GAP, Section 1.6).