3.4.2 Cyclic AMP
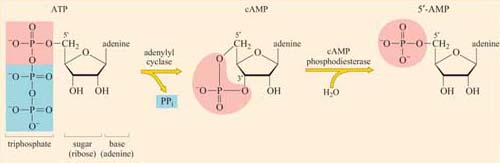
The concentration of cyclic AMP (cAMP) in the cytosol increases 20-fold within seconds of an appropriate stimulus. This is achieved by the action of the plasma membrane-embedded protein adenylyl cyclase, which synthesizes cAMP from ATP (Figure 34). cAMP is short-lived, as with all second messengers, because it is continuously degraded by cyclic AMP phosphodiesterases to 5′-AMP. Adenylyl cyclase is activated by stimulatory G proteins (Gαs) and inhibited indirectly by inhibitory G proteins (Gαi). It is usually the α subunit of the G protein that regulates adenylyl cyclase in this way, though sometimes the βγ complex can have the same effect. Different hormones can induce a similar cAMP response in a particular cell type, leading to similar cellular outcomes. However, different cell types will respond differently to similar cAMP increases, so the same hormone may have different effects on different cells. For example, cAMP mediates the action of adrenalin acting through β-adrenergic receptors (see Figure 48), causing glycogen breakdown in skeletal muscle cells, while promoting triglyceride breakdown in fat cells. Most of the downstream signals of cAMP are propagated by cAMP-dependent protein kinase A (PKA), which is a serine–threonine kinase.
PKA has many target proteins, which vary according to cell type. This explains why a hormone can produce the same increase in cAMP concentration in different cells, yet the cellular response to this increase is different for each cell type. PKA consists of two catalytic subunits and two regulatory subunits. The latter have two roles. They bind to PKA-anchoring proteins, thus tethering the enzyme to particular subcellular locations. They can also each bind two cAMP molecules in a cooperative fashion; when bound to more than two cAMP molecules, the regulatory subunits undergo sufficient conformational change for them to dissociate from the catalytic subunits (which remain in the cytosol). As with other signalling proteins with multiple binding sites, such as calmodulin, PKA is allosterically regulated by the second messenger cAMP. The released catalytic units are now active. They may act in the cytoplasm, for example by phosphorylating enzymes involved in glycogen metabolism and/or they may migrate to the nucleus, where they can switch on transcription of genes containing cAMP response elements (CREs) in their promoters. This is achieved by phosphorylating a serine on the nuclear CRE-binding protein (CREB), which, together with a coactivator, then binds to the CRE and stimulates transcription of the downstream gene.