Collagen: a fibrous protein
Collagen is the most important fibrous protein present in animals. In fact, collagen is the most abundant extracellular matrix protein of different tissues (reaching up to 30% of total body proteins in animals) providing structure, strength, and flexibility (Figure 3). Collagen fibers can be arranged differently, depending on the type of tissue and its biological function. In tendons, collagen fibers are arranged in parallel bundles (Figure 4).
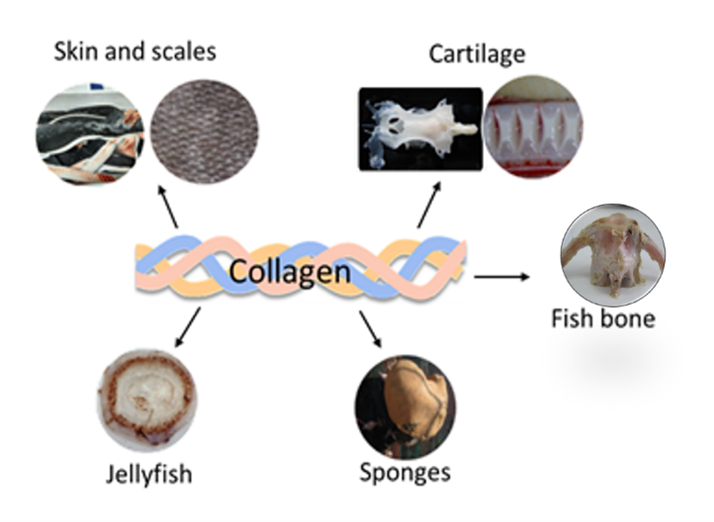
Figure
3.
Marine organisms and tissues containing collagen.
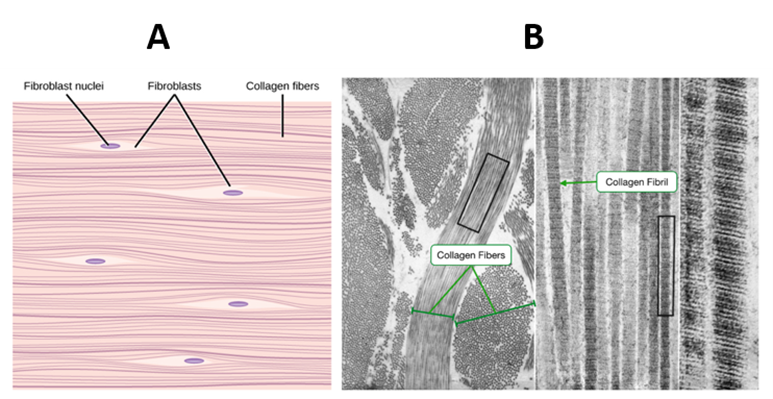
Figure 4. The parallel arrangement of collagen fibers showing fibroblast responsible for collagen synthesis (A). TEM image of a cross and longitudinally section of fibrils showing the characteristic repeating banded pattern (B).
Collagen is a complex supramolecular structure that self-assembles into cross-striated fibrils (Figure 5B and 5C). It presents 3 main characteristics:
a) The basic structure (monomer) presents a right-handed triple helix formed from three left-handed α-chains. Two of these chains present a similar molecular weight around 120 kDa (α1-chains) and the other (α2-chain) has around 97 kDa. This monomer also known as Tropocollagen has 300 nm long, 1.5 nm diameter, and 300 kDa of molecular weight.b) Presence of a triple amino acid repeating sequence in each polypeptide chain: Gly–X–Y, where Gly (Glycine) represents the 30% of all amino acid content and X and Y are with high prevalence Proline and Hydroxyproline.
c) Tropocollagen molecules assemble into complex structures leading to macroscopic fibers (Figure 5A) that are essential components of tissues and bones.
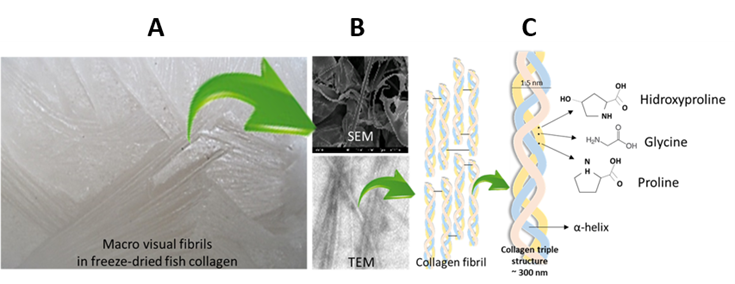
Figure 5. Freeze-dried fish collagen (A); Electron microscopy fibrils (B); Assembly of triple helix collagen structure (C).
Types of collagen: nearly 28 types of collagen have been described up to date1. Based on its macromolecular structures, collagen can be divided into four major groups (Figure 6):
- fibril-forming collagen including the major and abundant types I, II, and III collagen.
- basement membrane collagen including type IV.
- short-chain collagen including type VI, VIII, and type X.
-
other containing collagens with multiple
interruptions of their triple-helical Gly–X–stretches (FACITs).
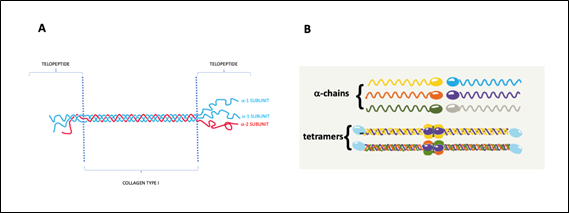
Figure 6. Fibril-forming collagen. Collagen type I2 (A); Basement membrane collagen. Collagen type IV3 (B).
Collagen fibers are responsible for the elastic and
viscoelastic properties of the tissues. One of the main
known functions of the collagen fibers is to take over the mechanical load.
- The mechanical strength or tissue stability is based on different parameters such as length and diameter of the collagen fibers, their spatial distribution, the collagen types present, the content of non-collagenous molecules (elastin, proteoglycans, etc.), and crosslinking content.
- During loading, the collagen hierarchical structure is deformed and fibrils can split into individual microfibrils. The collagen network breaks when several microfibrils break up, a process termed defibrillation.
- The deformation mechanism of collagenous structures is divided into four regions (Figure 7).
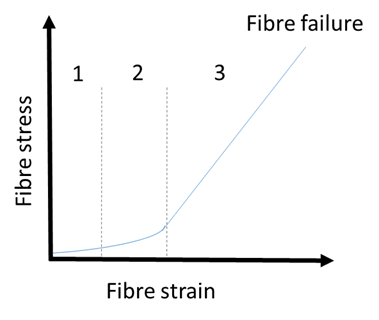
Figure 7. Typical stress-strain curve/deformation mechanism of collagen-based devices depicting the four distinct regions: the toe region (1), the heel region (2), the elastic region (3), and the failure region. Adapted from Shroushanova et al. 2019.
