Biochemical characterisation of collagen. Amino acid content and denaturation temperature
Physico-chemical characterisation of collagen is important to decide whether a biomaterial is useful for a particular application. The most relevant physicochemical analysis includes amino acid content, denaturation temperature, molecular weight, and hydrophobicity:
- Amino acids, as mentioned before, are the building blocks of proteins. Collagen is characterised by the high content of Glycine, Alanine and Proline, and Hydroxyproline (Table 2).
- Thermal properties of collagen are determined by differential scanning calorimetry (DSC). DSC can be used to analyse the thermal stability of collagen which is directly related to its amino acid content. Denaturation temperature is correlated with the habitat temperature, with cold-water fish having a lower denaturation temperature. Among fish living in the same habitat, there is a positive correlation between the denaturation temperature and the amino acid content (Proline and Hydroxyproline).
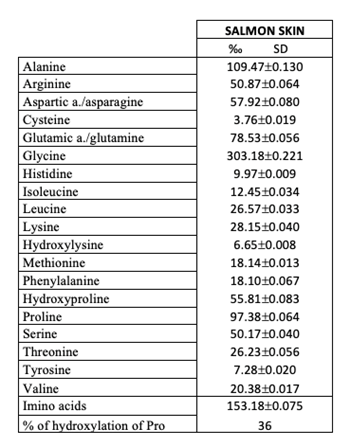
Table 2. Amino acid content (residues per 1000 residues ± Standard Deviation) of Type I skin collagen extracted from Atlantic salmon (Salmo salar) skin by-product.
