Biochemical characterisation of collagen. Molecular weight: SDS-PAGE and GPC-LS
Structural information of collagen such as purity, concentration, or crosslinking degree can be assessed using electrophoretic or chromatographic techniques. The SDS-PAGE (Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis) separates macromolecules such as nucleic acids or protein fragments according to their molecular weight or size, based on differences in electrophoretic mobility under an applied electrical field. In the case of collagen extracted from aquaculture by-products, different protein bands, such as α1 and α2-chains, β, and gamma components of higher molecular weight crosslinked components are subsequently visualised using Coomassie Brilliant Blue (Figure 9). In this figure can be observed that collagen from different species is similar and the α1 and α2-chains indicate the presence of type I collagen in all these subproducts. β and gamma components are also presents indicating the preservation of native characteristics of the collagen extracted.
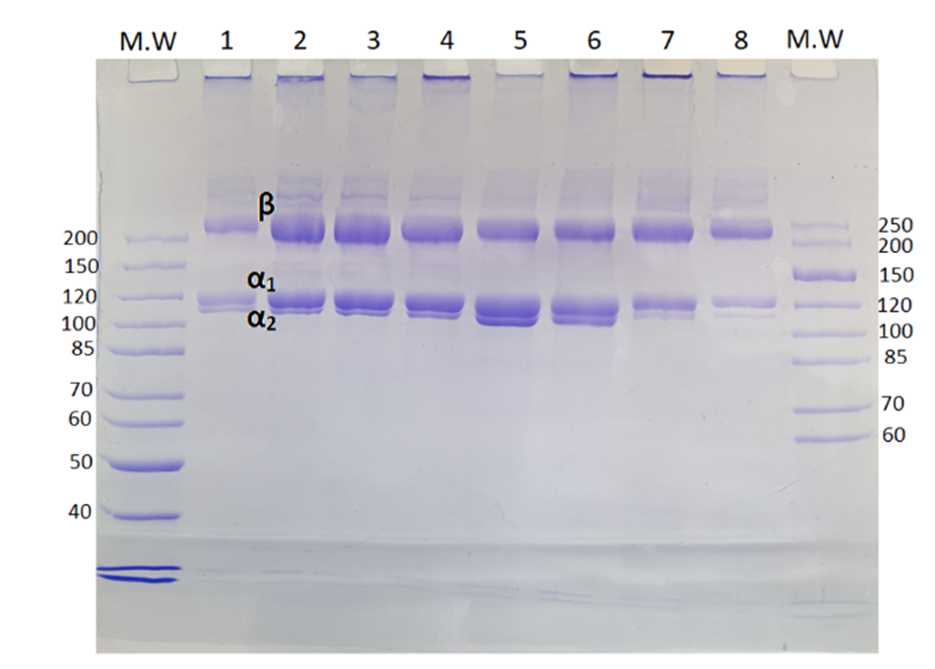
Figure 9. MW: molecular weight standard (kDa) 1. Salmon skin 2. Turbot skin 3. Turbot trimmings 4. Turbot head 5. Rainbow trout skin 6. Rainbow trout trimming and frames 7. Seabream skin 8. Seabass skin
Gel Permeation Chromatography coupled with a Light Scattering detector (GPC-LS) also provides molecular weight data and structural information regarding the presence of monomers (alpha-chains), dimers (beta components) or higher molecular weight crosslinked components (HMWCC) of collagen (Figure 10).
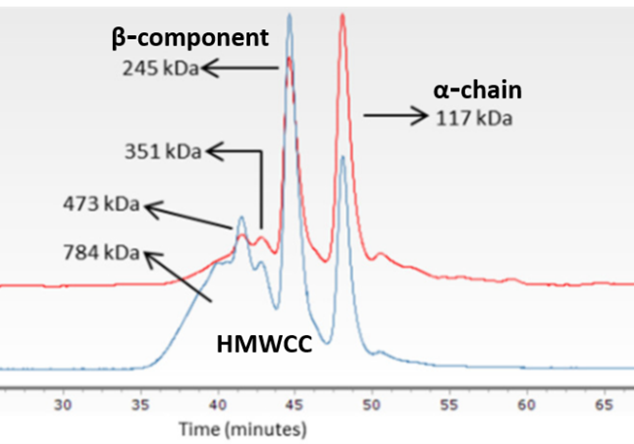
Figure 10. GPC-LS of type I collagen obtained from fish skin by-products. Red line indicates the refraction index value. Blue line indicates LS values.
