2.2 Chain repeat units
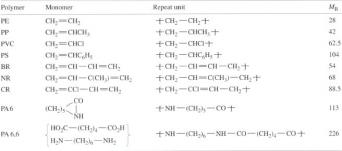
The repeat units of a range of polymers together with the monomer units from which they are derived are shown in Table 3. The simplest repeat unit is that for polyethylene, and consists of two carbon atoms linked to four hydrogen atoms. The difference between the monomer and the repeat unit is the loss of the double bond in the former to give the chain-linked repeating group. Thus the molecular masses of both monomer and unit are identical at 28. The molecular mass of the repeat unit is usually designated MR and is simply the sum of the atomic masses Ai of the component atoms (i) of the repeat unit:

|
The MR of polypropylene (PP) is (3 × 12) + (6 × 1) = 42. The number of repeat units in a chain is specified by the degree of polymerization, n , but a much more commonly used measure is the chain molecular mass M

Various types of polymer can be generated from similar monomer units, and Table 3 shows three families of closely related polymers. Replacing a single hydrogen atom in ethylene by a methyl group (—CH3) yields propylene, which when polymerized forms polypropylene (PP). When a chlorine atom (Cl) is substituted, the polymer is PVC and when a benzene ring (—C6H5) is substituted, the polymer is polystyrene. MR increases in this series, so that a polystyrene sample of molecular mass 10 000 will possess only 104/104 or roughly 100 repeat units compared to nearly 360 for a linear polyethylene of the same molecular mass. When just one hydrogen atom is substituted in the ethylene molecule, so-called vinyl polymers are created. When both hydrogen atoms on one of the two carbon atoms in the repeat unit are substituted, vinylidene polymers are formed. Thus poly(vinyl fluoride) has the repeat unit [CH2—CHF]n while poly(vinylidene fluoride) or PVDF has the repeat unit [CH2—CF2]n. Polymerization of tetrafluoroethylene monomer of structure CF2=CF2 yields PTFE ( Teflon ).
While most vinyl polymers are rigid thermoplastics at room temperature, introduction of a double bond into the repeat unit creates some of the common rubbers already described (see Table 1). The precursor monomer units are dienes, that is, they possess two double bonds as shown by BR and NR in Table 2.
A quite different way of constructing polymer chains is shown by the repeat units of polyamide 6 and 6,6 (nylon 6 and nylon 6,6). Here the monomer molecules are linked together by acid and amine groups at their ends. They are known as functional groups and play a significant role in controlling the physical properties of the final polymer. An important way of studying such functional groups, and other ways in which atoms are linked together in polymer chains, is spectroscopy (Box 3).
