3.3.1 Ethylene, propylene and butadiene
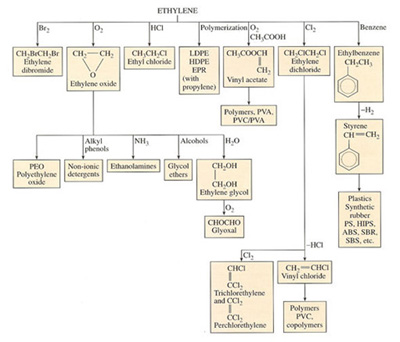
Nowadays ethylene is the most important building block for the chemical industry, particularly as a monomer in its own right, as a co-monomer with other vinyls, and as a source of vinyl monomers. It is the prime source for ethylene oxide, which is another major source of polymers, glycols and ethers. They can also be used to build up more complex C4 molecules and aromatics.
Some of the ways in which the ethylene molecule is modified to create other chemicals and polymers are shown in Figure 35. In terms of gross tonnage, the two most important are the reaction with benzene to form ethylbenzene and hence styrene, and the formation of vinyl chloride monomer by two steps with chlorine. PVA, a staple ingredient of emulsion paints and adhesives, comes from the monomer by a simple reaction with acetic acid and oxygen. In a similar way, vinyl chloride monomer is produced by a pathway which also yields chlorinated hydrocarbon solvents.
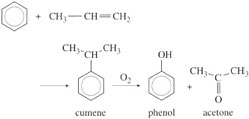
As a co-product of ethylene from steam cracking, propylene is a useful intermediate for building C3 monomers: propylene oxide, acrylonitrile and methyl methacrylate. Although the repeat unit of PMMA contains five carbon atoms, it is in fact produced from acetone and hydrocyanic acid (HCN). Acetone itself comes from an interesting reaction between benzene and propylene to produce cumene, which is then split to make phenol and acetone:
UK production of ethylene in the last twenty years has risen faster than that of propylene because of the greater usefulness of ethylene as a building block. This has been achieved by running crackers at higher severity (Figures 32, 33) to try to match market demand. This has not been totally successful, with the consequence that propylene prices have risen slower than those of ethylene.
Hydrocarbons with four carbon atoms are also produced in thermal cracking; the rate of production is related to the cracking severity and the type of feedstock used (Figure 33). Naphtha cracking produces large amounts of butene and only small quantities of butadiene, which is the more valuable component and is used in the production of synthetic rubbers. Nevertheless the amount produced is currently adequate to satisfy demand, mainly because the synthetic rubber industry has reached a mature plateau of production.