4.3.3 Termination and transfer
There are basically three ways in which chains terminate.
The first is known as coupling and occurs when two free radicals join together. This can be represented by the general equation
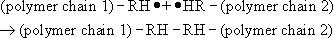
Such a mechanism significantly increases molecular mass, if it results in two polymer chains joining. This is the main mechanism which terminates the polymerization of styrene.
An alternative mechanism that may occur when two radicals interact is known as disproportionation. In this case, one molecule abstracts a hydrogen atom from the other and the other molecule forms a double bond
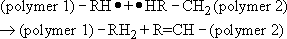
Disproportionation has no effect on molecular mass. Poly(methyl methacrylate) (PMMA) terminates by a mixture of coupling and disproportionation.
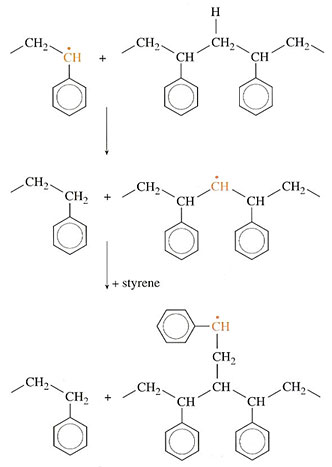
The third method of termination is chain transfer in which a radical abstracts a hydrogen atom from a neighbouring molecule. In the case of polystyrene the effect will be as shown in Figure 37, where (a) shows the situation before the interaction and (b) shows the structures after chain transfer in which the radical is transferred to one of the mid-chain carbon atoms. The new radical may now attack further styrene (Figure 37(c)) but, because it is not on the end of the chain, side branching occurs.
A similar mechanism accounts for the side branches in LDPE where it is a more important mode of termination than in polystyrene. Transfer to monomer; initiator or solvent (if present) can also occur in free radical polymerization, and effectively increases the dispersion of the molecular mass of the final polymer.
If termination is simply by disproportionation, then

where K is a constant, [M] the concentration of monomer, [I] the concentration of initiator, e.g. peroxide, and n the degree of polymerization. The square root arises because two free radicals react together during termination. If termination is by coupling there will be an extra factor of two in the constant compared to disproportionation. So the degree of polymerization or molecular mass can be controlled by varying monomer concentration – for example, by conducting the reaction in solvent – or by varying initiator concentration.
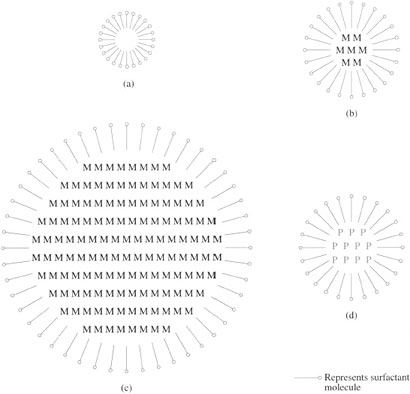
Controlling polymerizations on an industrial scale is of critical importance for molecular mass, and hence the processability and physical properties of the polymer, and one of the most important variables is the temperature of reaction. All polymerizations are exothermic (heat is liberated due to bond formation) and the heat must be conducted away to maintain a uniform reaction temperature. This is much more easily achieved when an inert solvent is used. Another method very commonly used industrially is to emulsify the monomer with a soap and conduct the reaction in water – so-called emulsion polymerization (Figure 38). Since control of molecular mass is so vital, extra aids are used industrially in addition to varying monomer and initiator concentrations. Reactions are ‘short stopped’ before all monomer is consumed by adding a specific chemical which reacts with free radicals, stopping them dead. Other chemicals can be added to induce transfer reactions, so controlling molecular mass distribution.