2.7.1 Thermosets
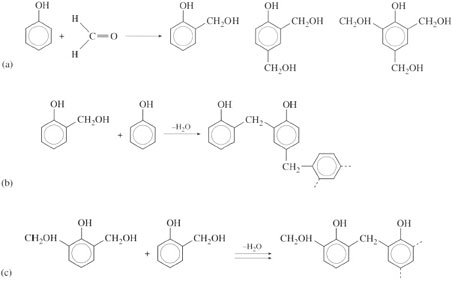
There are some limitations to the concept of the repeat unit when applied to crosslinked polymers, the thermosets. This is because of the complexity of the crosslinking reactions, the way molecules link together chemically during thermoset processing. For example, phenolic resins (the basis for materials like Bakelite) are prepared initially as prepolymers, i.e. polymers of low molecular mass (ca. 1000) by reaction between phenol and formaldehyde (Figure 31). Reaction can occur in several ways depending on the catalyst used and the ratio of phenol to formaldehyde: acid catalysis gives novolaks (Figure 31(b)) and alkaline catalysis produces resols (Figure 31(c)). The latter always possess free alcohol groups which are not present in novolaks. The prepolymers are blended with fillers before moulding to shape. With resols, no extra crosslinking agent is needed whereas multi-functional agents such as hexamine are added to novolaks to ensure crosslinking. Since the reaction occurs at three possible sites on the phenol molecule (Figure 31(a)), it is clearly not possible to specify a repeat unit in a simple way. The molecular mass is practically infinite since all parts of the polymer are linked together by chemical covalent bonds.
Self assessment question 5
Nomex is a polymer fibre with the following repeat unit:
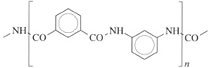
It is widely used in aircraft composites as a reinforcing fibre. From an examination of the structure, indicate whether you would expect the polymer to be crystalline. Briefly indicate what thermal properties you might expect from this material. Include mention of thermal transitions and fire-resistance by relating chain rotation to the effects of temperature on the structure.
Answer
Nomex has a repeat unit which cannot show any form of configurational isomerism so, provided there is enough energy to overcome any rotational barriers, should be crystalline. The barrier to rotation should be high, simply because the aromatic benzene ring is a fused structure, so any rotation that can occur is limited to the amide group (–NH–CO–). This shows hydrogen bonding with other amide groups, so the barrier to rotation should be very high, especially as there may be hindrance from the adjacent benzene rings. This implies that Tg, and Tm(since TgTm≈ 2/3) should both be high, considerably higher than the equivalent transitions of nylon 6.6 for example, the nearest analogue polyamide. By increasing such values, it will improve the fire resistance of the material owing to the presence of the very stable aromatic ring.
