3.2.1 Thermal cracking
The bulk of the major monomer and intermediate, ethylene (C2H4), is still produced in the UK by steam cracking without the use of catalysts. Paraffinic feedstocks are best for optimising ethylene yields, and the severity of cracking is specified by the rate of disappearance of a marker compound, usually n-pentane. The severity of the reaction can then be defined as follows:
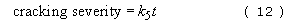
where k 5 is the rate constant (per second) for the cracking of n-pentane (C5) and t is the time in seconds. The rate of disappearance can be simply related to the degree of conversion, α, assuming first-order kinetics:
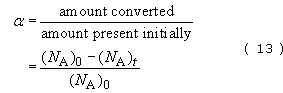
and

where (NA)0 and (NA)0 are the number of molecules (or moles) of compound A present at t=0 and t seconds respectively. Cracking severity is also dependent on temperature T through the Arrhenius equation
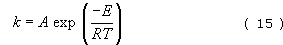
where E is the activation energy for the process, A and R are constants and k is the rate constant as in Equation (12).
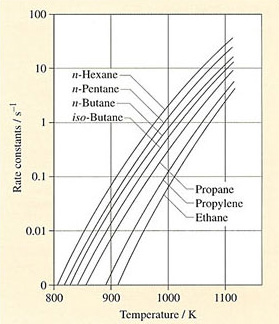
The exponential dependence of rate constants on temperature for a variety of simple hydrocarbons in thermal cracking is shown in Figure 32 and it is clear that large molecules are easier to crack than smaller ones. Ethane for example cracks more than 20 times more slowly than n-hexane at 1000 K. But what products are formed? Unfortunately, ethane can break down more easily to methane and hydrogen and ultimately to carbon, particularly under the rather crude conditions of thermal cracking. Propane and higher homologues give higher yields of ethylene under similar cracking conditions but cyclic paraffins (cycloalkanes) give rather less.
The fractionated naphtha is divided into two streams, one to the thermal cracker (olefin plant) and the other to the reformer (aromatics plant). The exit streams are separated and individual chemicals subjected to separate treatments depending on the intermediates, monomers or polymers required.
A highly paraffinic naphtha is thus the best cracking feedstock for high yields of ethylene, and Figure 33 shows the kind of product distribution from a Kuwaiti naphtha under various cracking conditions. The major co-products – ethylene, propylene, butadiene (C4H6), mixed butene/butane (C4H8, C4H10) and pyrolysis gasoline (C5+) − vary in concentration depending on cracking severity. With naphthas derived from other crudes, the product distribution will be quite different (see Box 7). The proportion of unwanted byproducts like hydrogen, methane and carbon coke (not shown in the figure, but which gradually accumulate within the cracking tubes) increases with cracking severity. However, the product distribution may not coincide with polymer demand and consumption of ethylene for other chemicals.