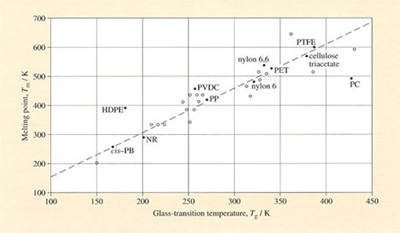
2.5.4 Melting and structure
For those polymers which can crystallise, one would expect some relation between chain rotation and melting. Since all crystallisation demands that chains form an ordered conformation (e.g. the PE planar zig-zag) before they can pack together, the chance of this happening should be related to the ease of twisting into the required conformation. That there is a rough correlation between Tg and Tm can be judged from Figure 12, where the two transition temperatures are plotted against one another. Averaging over the scatter of points shows that when the transitions are plotted in kelvins, then
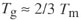
Some care is needed in interpreting such a rule of thumb, and there are known to be other factors at work, such as hydrogen bonding as we've already seen above in polyamides. But overall, there does indeed appear to be a correlation between ease of chain rotation and the most important thermal transitions observed in polymers. Such relations can be a guide to synthesis of new repeat units.