4.5 Copolymerization
The alloying of metals to improve their properties is widespread and although many polymers used today are relatively pure (e.g. polystyrene, nylon), an increasing number are mixtures of two or more polymers. As with metals, one reason for doing this is to increase the range of properties. The major practical problem, however, is that homopolymers blend together with difficulty and even where blends are possible, as in some thermoplastics, phase separation can occur readily.
This problem is often overcome by polymerizing a mixture of monomers, a process known as copolymerization. It gives a much greater range of structures than is possible by mixing homopolymers because of the possibility of branching, structural isomerism within a single monomer, and the way in which the different repeat units can be added together. In addition, composition can be varied over very wide limits and, of course, molecular mass can be varied to achieve the desired balance of properties in the final product.
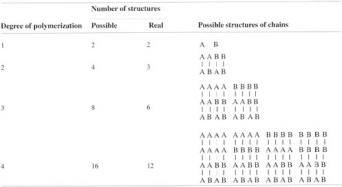
One central problem of copolymerization is manipulation of the order of repeat units along the length of the chain. To illustrate this, suppose that two monomers, A and B, are copolymerized. The chain could start with either a molecule of A or a molecule of B, and at each successive addition there are always two possibilities as to which monomer molecule will be attached. As shown in Table 7, the number of possible chain structures grows rapidly as n increases. Since the number of possible structures is proportional to 2n, it is easy to see that even for low degrees of polymerization the number of possible copolymers is very large indeed. Some of these molecules are identical however (AB is the same as BA for example), so that the number of real structures will be somewhat lower, as shown in Table 7.
Box 9 Making nylon
Nylon is a familiar polymer both as a fibre (in textiles and ropes), monofilament (in fishing lines and toothbrushes) and in mouldings (such as the plugs on large screws for making attachments to brick and concrete walls). It was first made in the USA in 1936/7 by a university chemist employed by DuPont, W.H. Carothers by name. He more than any other scientist opened up the then obscure subject of polymers to commercial exploitation. He was also closely involved in the development of one of the most important synthetic rubbers, polychloroprene (Neoprene) during the same period. In April 1937, the first experimental nylon 6,6 stockings were made, followed rapidly by full plant production in 1938 after a good reception from the (female) consumer. The stockings, for example, proved cheaper and more durable than the silk stockings which then dominated the market. Most nylon however, was produced for parachutes, tyre cord, and rope for military use in the Second World War, which then intervened. The Germans competed by making nylon 6 in 1941–2, and the two types still compete in the market.
The industrial production of nylon (or aliphatic polyamide) is beset with problems, however. An important practical consequence of step growth behaviour, for example, is that small amounts of impurity can seriously inhibit the growth of high molecular mass polymer. To counter this problem, monomers must be purified carefully and, in the case of the nylons, the monomers must be in such a form that the numbers of different functional groups are exactly equal:
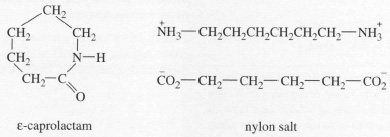
Nylon 6 is prepared by breaking the ring in ε-caprolactam and nylon 6,6 by heating nylon salt, when the ionic structure breaks down to form linear chains. In both cases, the amine and acid groups are of equal concentration. With other step growth polymers, there are similar problems, prepolymers often being made to overcome the stringent requirements of the process.
|
In fact, the structures which form are not entirely random if the reaction is started with a particular mixture of monomers. Table 7 shows that the composition varies from chains of only monomer A (homopolymer A) to chains containing only monomer B (homopolymer B). Between these two extremes it is possible to identify structures of the type AAA… BBB… where there are relatively long sequences of either A or B monomer units; these are known as block copolymers. There are also structures of the type ABAB… known as alternating copolymers (see section 2.2.6).
In many of the structures no regularity can be detected, although there will be short sequences of one type of unit, and the copolymer can be regarded as completely random; such copolymers are usually said to be ideal copolymers. These possible copolymer structures are shown schematically in Table 7.
