4.5.2 Commercial copolymers
The main reason for copolymerizing different monomers is to adjust the physical properties of a given homopolymer to meet a specific demand. SBR elastomer, for example (Table 1), based on 24 wt% styrene monomer shows better mechanical properties and better resistance to degradation than polybutadiene alone. By increasing the styrene content to 35 per cent, a high hysteresis (energy absorbing) material ideal for tyre treads is produced. Another example is nitrile rubber, which is produced by a free radical emulsion copolymerization of butadiene and acrylonitrile to make an oil-resistant rubber suitable for oil and petrol lines.
A second reason for copolymerization is to enhance the chemical reactivity of a polymer, particularly to aid crosslinking. Conventional vulcanization in rubbers is brought about by forming sulphur crosslinks at or near double bonds in the chain. In polyisobutylene where the main chain repeat unit is
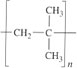
there are no such bonds. So the isobutylene monomer is copolymerized with a few weight percent isoprene units to make IIR (butyl rubber) which can be vulcanized easily.
This is also the reason why EPDM rubber consists of no less than three different monomer units copolymerized together (ethylene, propylene and a diene) using Ziegler-Natta catalysts. The copolymer structure is random, so crystallinity is low and the material behaves like a rubber when vulcanized across the diene double bonds.
To show the dramatic effect of copolymer structure on physical properties, consider the change from random SBR copolymer to a block copolymer of exactly the same chemical composition but where the styrene and butadiene parts are effectively homopolymer chains linked at two points:
![]()
The material behaves like a vulcanized butadiene rubber without the need for chemical crosslinking since the styrene chains segregate together to form small islands or domains within the structure. Such so-called thermoplastic elastomers (TPEs) today form an important growth area for new polymers because of the process savings in manufacture that can be achieved with their use.
Among rigid thermoplastics, the most widely used copolymers are those of styrene and they include ABS, HIPS and SAN. Both HIPS and ABS are graft block copolymers where the elastomeric side chains are deliberately introduced to improve the toughness of the material (see section 2.2.6).
Self assessment question 8
What are the structures of the following free radical polymerized copolymers
-
vinyl chloride-vinyl acetate copolymer containing 10 per cent vinyl acetate,
-
SBR containing 24 per cent styrene,
-
SAN containing 76 per cent styrene?
Answer
The copolymer structures can be determined by referring to the reactivity ratios of the relevant monomers (Table 8).
(a) For vinyl acetate reacting with vinyl chloride, the reactivity ratios are

The product r1 r2 = 0.39. This value is less than 1, so the structure will be random with a pronounced tendency for alternation between the repeat units of each monomer in the chain. Both repeat units are potentially stereoisomeric but atactic structures are usually formed in free radical polymerization so that the copolymer is non-crystalline.
(b) SBR approaches the ideal or random copolymer structure most closely because r1 r2 = 0.78 × 1.35 = 1.05. The butadiene repeat unit is capable of geometrical isomerism, with three possible structures: cis, trans and vinyl. These are randomly distributed through the chain structure owing to the unspecific nature of free radical polymerization.
(c) Styrene-acrylonitrile (r1 r2 = 0.016) is very close to being an alternating copolymer with the repeat unit
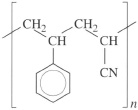
This material would have a molar composition of 50 per cent styrene, but taking the different MRs into account (styrene = 104, acrylonitrile = 53) the weight composition is 66 per cent styrene, not far removed from the actual composition of 74 per cent styrene. Both repeat units are atactic, so the material is non-crystalline.
