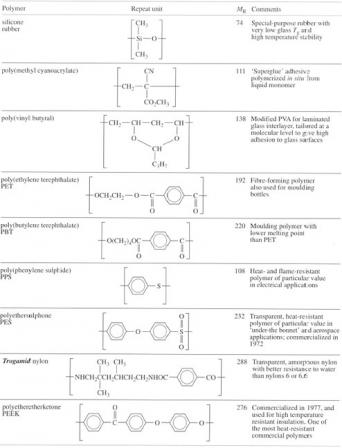
2.7 Commercial polymers
The increasing control of polymer structure by fine-tuned catalysis of polymerization opened up an enormous area for commercial exploitation, and new polymers are still being produced in this way (such as the metallocene polymers). A revolution of equal magnitude has occurred with polymers containing functional groups, for example, the nylons, polyesters and polyurethanes, resulting in polymers ranging from quite simple structures like aramid fibre to relatively complex repeat units like those in polyimide or polycarbonate. These polymers are mainly based on carbon-carbon, carbon-oxygen or carbon-nitrogen bonds, but a significant amount of research is also being put into polymers like the silicones (Table 6) which are termed ‘inorganic’ (Box 6). Silicone polymers have been known since the 1940s and were developed for both low and high temperature resistant seals for aircraft engines, although many other uses were found for their water-repelling properties. A number of speciality silicones now exist, mainly varying in molecular mass and the pendant organic side groups.
Polymeric adhesives were traditionally based on concentrated solutions of latex rubber, PVA or epoxy resins, but a novel approach developed in the sixties involved direct polymerization of cyanoacrylate monomer at the surfaces to be bonded. The main advantage is ease of application of the liquid monomer to the surfaces, where its low viscosity enables it to penetrate into the finest crevices. The monomer polymerizes very rapidly by contact with traces of water and gives a very strong bond. It is now finding widespread use in industrial assembly as well as for domestic purposes. Poly(vinyl butyral) is another speciality adhesive primarily used for bonding glass sheets together to form laminated or safety glass. PVA is partially reacted with butyraldehyde to make the adhesive which is then bonded to the glass surfaces. In service, it acts to stop crack growth penetrating from one glass sheet to the next by absorbing energy like a rubber.
|
Box 6 Inorganic polymers
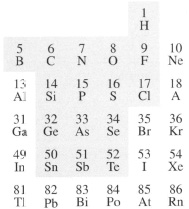
Silicone rubber is an example of an inorganic polymer, one member of a very large family of inorganic materials which have a chain or sheet molecular structure. Elements which can link together with themselves (‘catenation’) are not limited to carbon, with nitrogen forming a dimer (N2 gas), phosphorus in its coloured forms (Pn), plastic sulphur (Sen) and amorphous selenium (Sen ). Generally, it is those non-metals or metalloids lying near carbon which catenate, as highlighted in Figure 30. The polymers formed by linkage with another element are much more common, however, silicon being an element which forms extremely strong bonds with oxygen (Si-O) in many silicate minerals, for example. Chain formation in inorganic silica-based glasses is shown by the amorphous, non-crystalline nature of the material (transparent window glass, for example) and the high viscosity of molten glasses. The strength of Portland cement derives from the formation of a polymerized calcium silicate formed during setting.
Phosphorus also forms very strong chemical bonds with oxygen, frequently forming long chain polymers. Indeed. DNA, the stuff of life itself, possesses a backbone chain which is a copolymer of phosphate groups (PO4) with sugar molecules.
Functional groups which form part of the main chain offer very great flexibility in synthesis not only because of the variety which exists but because of the very large number of possible structures that can be linked together. The selection shown in Table 6 includes staple polymers like PET, which has recently received a boost through development of blow moulding grades, but also high technology materials like PEEK. Traditional bulk polymers like the polyolefins, PVC and PS are limited in application particularly for engineering use by virtue of their low melting or glass transition temperatures. The concept of rotational isomerism stimulated research into ways of stiffening chains by the introduction of aromatic groups. Outstanding success stories include polycarbonate, a tough transparent thermoplastic discovered in 1954 and available commercially in the early sixties, Kevlar aramid fibre (1968) and the polysulphones. They show progressively increasing temperature resistance: PC has a Tg of 145 °C, but polyethersulphone a Tg of about 230 °C. Polyetheretherketone (PEEK) exhibits a Tg of 144 °C but melts at 335 °C. These polymers are also highly resistant to burning, a feature which reflects the chemical stability of the aromatic benzene rings. It is a characteristic which reaches its ultimate in refractory graphite, which can resist temperatures above 3000 °C. Thus PPS has an oxygen index of 0.53 (the fraction of oxygen in air needed before burning will occur) compared to most common polymers which have indices less than 0.21, which is the normal oxygen content of the atmosphere. Introduction of aromatic rings into the nylons has similar effects, stiffening the chain and providing chemical stability. It is seen most dramatically with aramid fibre, and to a lesser extent with Trogamid nylon (Table 6). This polymer has a high Tg of about 150 °C yet is completely non-crystalline, and thus transparent, owing to the disruption of the crystal lattice by the irregular aliphatic diamine chain. The T g of nylon 6 or 6,6 is about 0 °C but varies with the amount of water absorbed in the material.
