3 Returning to different models to help understanding
In learning about carbon and its compounds, your students use a range of ways of representing information about molecules. One recurring model is the electron dot structure model resulting from carbon having four valence electrons. Structural diagrams are another way of encapsulating the same information about ‘where the bonds are?’. Every time a new type of compound or reaction is introduced, students need to go back to one or more models of molecular structure, to refresh, recall recent concepts and to scaffold a new understanding.
For a great deal of the time, one or both of these two-dimensional approaches may be sufficient for your students, but occasionally it will be helpful to remind your students of the three-dimensional nature of the molecules by using physical models.
When learning about soaps and detergents, your students may be puzzled why the structures called micelles are shown as zig-zags with a Na+ on one end. Why a zig-zag? Well, it’s a convention. Yes, but why this convention? Using physical models of alkanes with increasing numbers of carbon atoms makes it very evident that the ‘spine’ of a carbon compound is more like a zig-zag than a straight line.
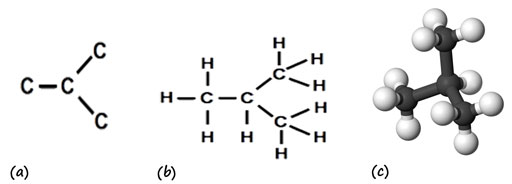
Using physical models can help to remind students that diagrams only represent some aspects of a molecule’s structure, but can be actively misleading. The diagram of the branched structure for C4H10 in Figure 4.8 in the textbook and the related carbon skeleton in Figure 4.7 suggests that two of the carbon atoms are closer together, for example, whereas a physical model would show that the skeleton is a tetrahedral structure with rotational symmetry about more than one axis (Figure 5).
Sometimes, a combination of approaches is useful to help students to understand what is happening in a reaction. For example, students learn that one of the reactions of ethanoic acid is with an alcohol to produce an ester and water. There are several ways that you could examine this reaction with your students:
- Generalisation and prediction. This reaction is an example of esterification. As ethanoic acid is one of a homologous series of carboxylic acids, the reaction will always produce an ester plus water (Table 1).
| Reactants | Products |
|---|---|
CH3COOH + CH3CH2OH (ethanoic acid) (ethanol) |
CH3COOCH2CH3 + H2O (ester) (water) |
CH3CH2COOH + CH3CH2OH (propanoic acid) (ethanol) |
CH3CH2COOCH2CH3 + H2O (ester) (water) |
- Examining the reaction by using diagrams of the molecular structures (Figure 6).
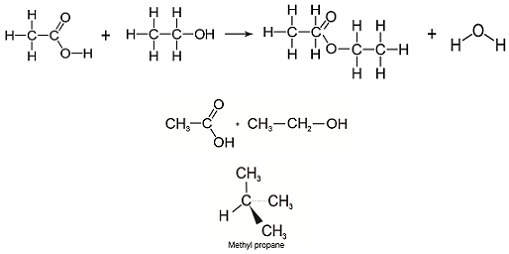
Using this approach can help to make it more clear what the molecular structures are.
- Focusing on the functional groups, the oxygen of the alcohol functional group attaches to the carbon of the carboxylic acid group (Figure 7).
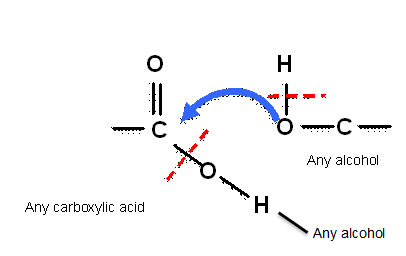
This is a more abstract approach, but shows ‘where’ the reaction happens and emphasises that the rest of the reactant molecule is unchanged.
- Using physical models of the reactants, then demonstrating how these molecules combine to produce the product molecules. This makes it clear that the hydrogen is lost from the alcohol functional group and combines with the –OH group from the carboxylic acid to make water. For some students, seeing the reaction happen using models may help them to remember the process.
Each of these models provides a different way of viewing the same event.
Pause for thought
|
Activity 3: Teaching the chemical properties of carbon compounds
This activity will help you to develop your planning and in-class teaching about the chemical properties of carbon compounds. You may find it useful to carry out this activity as a discussion with a colleague.
- Identify one aspect or section of the topic that your students are likely to find difficult.
- What are the key concepts that you want your students to remember about this section?
- What mental models or concepts do your students need to draw on in order to understand it? Which of these have students met and used before?
- Do you need to help them develop any additional mental models?
- How will you present this to your students?
- Will you use electron dot models, diagrams of molecular structures or physical models?
- What question will you use to direct your students’ attention to the important details?
- How can you increase student participation in the lesson?
- Can you ask your students to draw diagrams on the blackboard?
- And to explain reactions to your students?
- Could you ask your students to try to answer each other’s questions about this topic?
2 Using mental models to generalise and predict